Relationship between upregulation of CD40 system and restenosis in patients after percutaneous coronary intervention1
Introduction
Inflammation plays a pathogenic role in restenosis after percutaneous transluminal coronary angioplasty[1]. Recently, increasing evidence have shown that the CD40-CD40 ligand (CD40L) interaction plays a crucial role in the pathogenesis of atherosclerosis and coronary artery disease[2]. Furthermore, we found that patients with acute coronary syndromes (ACS) had higher serum concentrations of soluble CD40L than healthy volunteers or those with stable angina, and an obvious correlation was also observed between soluble CD40L (sCD40L) concentration and complex coronary stenosis[3,4]. Recently, the increasing CD40-CD40L system has been observed in patients with hypercholesterolemia, and the circulating sCD40L has strong independent prognostic value among ACS[5,6]. However, the role of the CD40-CD40L system in the pathophysiology of restenosis is still unclear.
Until now, there has been little information on whether the CD40-CD40L system may influence inflammatory reaction after vessel injury, which is ultimately responsible for luminal renarrowing after percutaneous coronary intervention (PCI) in humans. We hypothesized that the CD40-CD40L interaction might also contribute to the development of restenosis after PCI. Therefore, the present study was designed to investigate the possible role of the CD40-CD40L system in restenosis after PCI and its correlation with the C-reactive protein (CRP), as well as lumen loss in PCI patients.
Materials and methods
Reagents The mouse anti-human CD40, mouse anti-human CD40L, and CD61 fluorescein isothiocyanate (FITC)-conjugated antibodies were bought from PharMingen (San Diego, CA, USA). sCD40L, an ELISA kit was purchased from Bender Medsystems (PharMingen).
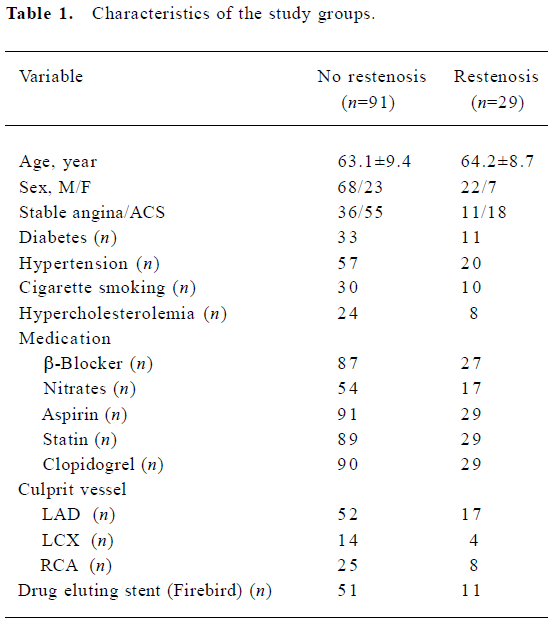
Patients and controls One hundred and twenty patients with stable angina or ACS were enrolled in the trial. Coronary angiography showed that all the patients had substantial coronary artery disease with a diameter stenosis of at least 70% at a culprit lesion that was suitable for angioplasty. Drug-eluting stents were implanted in the patients with diabetes or in the patients with the ≤3.0 mm target vessel diameter or the >20 mm lesion length. For the comparison, 20 sex- and age-balanced donors served as a control group. Patients with infection, tumor, liver or kidney diseases were excluded. Informed written consent was obtained from each patient and the study was approved by the Ethical Committee on Human Research. We also excluded patients who were treated with anti-inflammatory drugs prior to enroll-ment, for example, statins, clopidogrel, and angiotensin II type 1 receptor blockers (Table 1).
Full table
Angioplasty procedure and follow-up evaluation PCI and quantitative coronary angiography were performed according to standard techniques as described previously[7]. Patients were followed for 6 months for restenosis control. Restenosis was defined as a loss of 50% or more of the initial gain during the follow-up period by angiography. The continuous luminal loss was defined according to the following equation: late loss=[(post-intervention minimal lumen diameter-follow-up minimal lumen diameter)/vessel size]×100%. The vessel size is the value of the reference diameter function at the minimal position of the obstruction.
Blood sampling protocol A rich-plasma platelet was prepared as previously described[8]. Briefly, peripheral venous blood was drawn into tubes containing sodium citrate. Citrated blood samples were centrifuged at 200×g for 10 min at room temperature to obtain platelet-rich plasma. Non-citrated blood was immersed in melting ice and allowed to clot for 1 h before centrifugation (1500×g for 10 min at 4 oC). The supernatant was stored at -80 oC until analysis. Samples were thawed only once.
Detection of CD40 and CD40L on platelets by flow cytometry Platelet immunostaining was performed as previously described[9]. Fixed blood was diluted at 1:100 with PBS and incubated with the first antibody (30 min at 4 oC) diluted with PBS. Then the platelets were incubated with PE-conjugated secondary antibody (30 min at 4 oC) and analyzed using CELLQUEST software (BD Bioscience, Bredford, MA, USA). For each treatment, the mean fluorescence intensity (MFI) value for the control stained population was subtracted from the MFI value of the positive-stained sample. Platelets were identified by their positivity for GP IIIa antigen (CD61) and their characteristic light scatter. The platelet population evaluated was ≥98% positive for CD61.
Enzyme immunoassays Serum and plasma samples were frozen and thawed only once. Specific immunoassays for sCD40L (sCD40L detection limit, 95 pg/mL; Bender Med-systems, PharMingen) and high-sensitive CRP on an IMMULITE automated analyzer (Diagnostic Product Corporation, Los Angeles, CA, USA) were performed in triplicate as previously described[7]. At our laboratory, the intra-assay and interassay coefficients of variation were less than 5%.
Statistical analysis Statistical evaluations were performed with Graphpad software (Prism 3.0, La Jolla, CA, USA) and SAS 8.0 software (SAS Institute Inc. Cary, NC, USA). Data were expressed as mean±SD and statistically compared by repeated measures. Correlation was evaluated through a regressive analysis. The Pearson two-way test and the Spearman two-way test were used to assess the relationship between 2 quantitative variables with normal or abnormal distribution. All P values of less than 0.05 were considered statistically significant.
Results
Clinical follow-up results No patients developed myocardial infarction or sudden death during the 6 month follow-up period. Restenosis occurred in 29 of the 120 PCI patients (24.2%). The incidence of restenosis was 17.7% and 31.0% in patients who received drug eluting stents or bare mental stents, respectively.
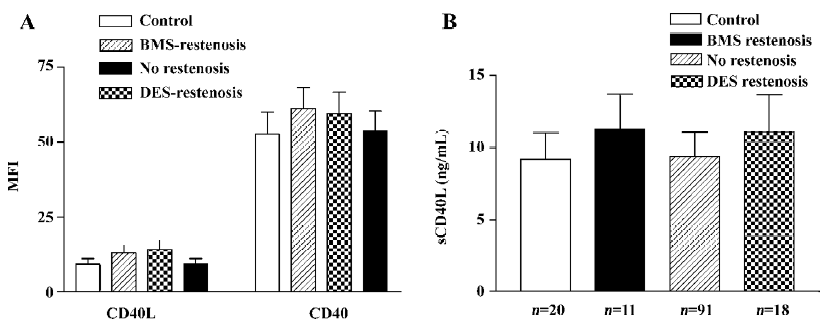
CD40-CD40L system expression Restenotic patients who received either drug eluting stent or bare metal stent showed higher levels of CD40 (MFI: 60.1±6.0 vs 54.1±6.3) and CD40L (MFI: 11.2±2.8 vs 9.2±1.8, P<0.01) expression on platelets as well as sCD40L (11.3±2.4 vs 9.4±1.6 ng/mL, P<0.01) compared with preoperative non-restenotic patients (all P<0.01; Figure 1A, 1B).
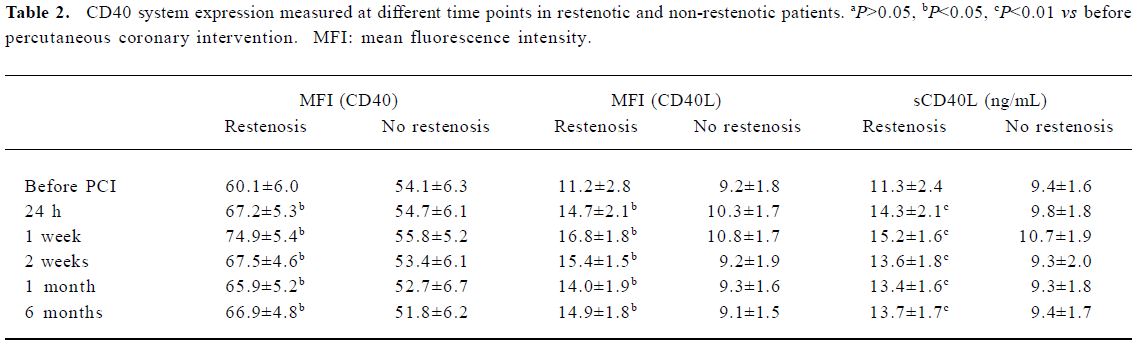
Furthermore, the CD40-CD40L system was analyzed in all patients preoperatively and postoperatively (24 h, 1 week, 2 weeks, 1 month, and 6 months after PCI). Persistent increases in the CD40 system were observed in restenotic patients after PCI during the whole 6 month follow-up period (P<0.01 vs before PCI), whereas they were normalized at 2 weeks after PCI in the non-restenotic patients (Table 2).
Full table
Relationship between CRP levels and CD40 system Patients who developed restenosis showed significantly higher CRP levels (1.48±0.34 vs 0.92±0.19 ng/mL, P=0.0001) than non-restenotic patients. Moreover, the CRP kept considerable levels in the restenotic patients during the whole follow-up period (Figure 2). The level of CRP was positively correlated with expression of sCD40L and CD40L in restenotic patients at different time points (Table 3). But it was not associated with the expression of sCD40L and CD40L in non-restenotic patients. The level of CRP had no correlation with CD40 expression for all patients.
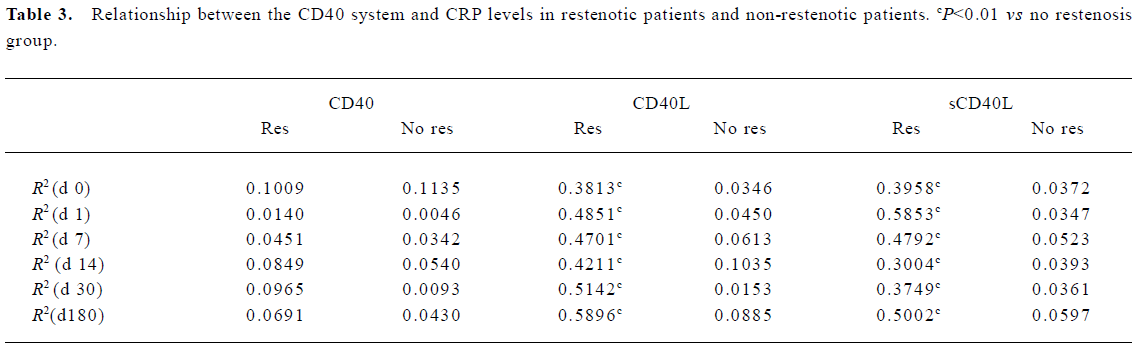
Full table
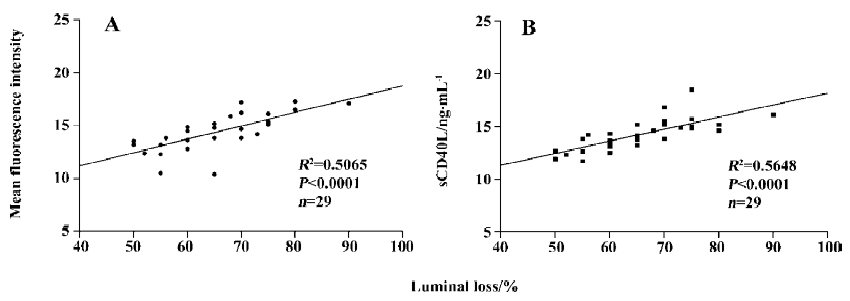
Correlation of lumen loss with CD40 system in reste-notic patients To investigate whether the CD40 system contributes to luminal restenosis after PCI, we assessed the association between the expression of the CD40 system and the degree of luminal renarrowing. The levels of the CD40 system were measured for 6 months after PCI. Lumen loss showed a significant positive correlation with sCD40L and CD40L by 6 months (Figure 3). However, there was no significant correlation of luminal loss with the expression of CD40 on platelets in the patients (R2=0.02513, P=0.3418, n=19).
Discussion
Restenosis is a serious complication of PCI[10]. PCI activates platelets and white cells and triggers an acute inflammatory response that plays a major role in the pathogenesis of complications. Increasing evidence support the role of inflammation in the restenosis after PCI[11]. Recently, Cipollone et al[12] reported that the pre-procedural level of soluble CD40L was associated with late restenosis after percutanerous transluminal coronary angioplasty (PTCA). We found that PCI resulted in significant increases in CD40, CD40L, sCD40L, and CRP in a short time for all patients, and patients who developed restenosis showed sustained higher levels of CD40L, sCD40L, and CRP than non-restenotic patients.
Platelets are major contributors to the elevated level of circulating sCD40L in patients with acute coronary syndrome and hypercholesterolemia, and more than 95% of the circulating sCD40L exists in platelets[13,14]. We demonstrated that the increased circulating sCD40L was from activated platelets in hypertension patients[15]. The disrupted endothelium by PCI resulted in the exposure of thrombogenic surfaces that support the adhesion, activation, and aggregation of platelets. Therefore, the platelet-rich thrombi may be the major intravascular source of enhanced expression of the CD40 system both on the platelet surface and in the circulating environment as they shed sCD40L.
CRP is an acute-phase reactant and a strong marker for inflammation. Elevated hs-CRP levels have a close correlation with the occurrence of restenosis or major cardiovascular events[16,17]. Most studies have focused on the importance of elevated hs-CRP levels before PCI and immediately after PCI. Our study showed significantly sustained high hs-CRP levels in restenotic patients. This result indicates that patients with long-term enhanced levels of CRP have a higher incidence of restenosis.
The CD40 system is a predictor of enhanced inflammatory response. Considerable evidence implicate that the CD40-CD40L interaction plays a crucial role in multiple stages of atherosclerosis[18]. Recently, Turker et al[19] reported that an increased pre-procedural sCD40L level was an independent predictor of stent restenosis during the 6 month follow-up period. Therefore, we suspected that the CD40 system was associated with restenosis after PCI. In this study, we demonstrated that the CD40L expression was not only significantly correlated with CRP levels, but also with the luminal loss in restenotic patients. This result may indicate an association between CD40L changes and the occurrence of restenosis. It also suggested that CD40L was a reliable risk predictor for restenosis and provided a potential preventive strategy against restenosis, such as using anti-CD40L antibody to prevent lumen renarrowing after PCI. Further large-scale cohort studies should be performed to illustrate the clinical implication of expression of the CD40 and CD40L system in patients after PTCA.
In this study, it was interesting to note that there was a correlation between restenosis and CD40L expression rather than the CD40 levels. This result was similar to our previously findings in which the increased CD40L levels indicated a significantly increased risk of major adverse cardiovascular events[6]. This interesting phenomenon may not only be due to the cause of activated platelets releasing circulating sCD40L in these patients, but also previous in vitro and in vivo studies which demonstrated a crucial role of CD40L in multiple stages of atherosclerosis and ACS[20]. Further study remains to be elucidated.
Limitations of our study were the small number of cohort patients and the limited sample collecting time which may have affected the results. Therefore, further large-scale studies and prolonged follow-up should be performed to illustrate the clinical value of CD40L levels independently or in combination with other markers for predicting the risk of restenosis. Simultaneous assessment of the CD40 system and other factors yields independent and complementary prognostic information and thus enabled a more powerful prediction of restenosis.
In conclusion, CD40L expression may provide a useful marker for risk assessment of restenosis after PCI. However, the precise role of the systemic inflammation in restenosis remains to be determined.
References
- Salgado FW, Martinez FE, Horta P, Lemos PA, Migueletto BC, Serrano CVJ, et al. Intracoronary inflammatory markers after percutaneous coronary interventions. Arg Bras Cardiol 2005;85:180-5.
- Phipps RP. Atherosclerosis: the emerging role of inflammmation and the CD40-CD40 ligand system. Proc Natl Acad Sci USA 2000;97:6930-2.
- Yan JC, Wu ZG, Li L, Zhong RQ, Kong XT. The clinical implications of increased expression of CD40L in patients with acute coronary syndromes. Chin Med J 2002;115:491-3.
- Yan JC, Wu ZG, Kong XT, Zhong RQ, Zhan LZ. Relation between upregulation of CD40-CD40 ligand system and serum levels of matrix metalloproteinases and complex stenosis morphology in patients with acute coronary syndrome. Acta Pharmacol Sin 2004;25:251-6.
- Yan JC, Wu ZG, Li L, Zhong RQ, Kong XT. The relation between expression of CD40-CD40 ligand system and serum cholesterol levels in patients with hypercholesterolemia. Chin Med J 2004;117:1101-3.
- Yan JC, Zhu J, Gao L, Ma GS, Wu ZG, Kong XT. The effect of elevated serum soluble CD40 ligand on the prognostic value in patients with acute coronary syndromes. Clin Chim Acta 2004;343:155-9.
- Cipollone F, Marini M, Fazia M, Pini B, Iezzi A, Reale M, et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol 2001;21:327-34.
- Yan JC, Wu ZG, Chen JM, Li L, Kong XT. Upregulation of CD40-CD40L ligand system in patients with diabetes mellitus. Clin Chim Acta 2004;339:85-90.
- Michelson AD, Barnard MR, Krueger LA, Frelinger AL III, Furman MI. Evaluation of platelet function by flow cytometry. Methods 2000;21:259-70.
- Kivela A, Hartikainen J. Restenosis related to percutaneous coronary intervention has been solved? Ann Med 2006;38:173-87.
- Bhatt DL, Topol EJ. Need to test the arterial inflammation hypothesis. Circulation 2002;106:136-40.
- Cipollone F, Ferri F, Desideri G, Paloscia L, Materazzo G, Mascellanti M, et al. Preprocedural level of soluble CD40L is predictive of enhanced inflammatory response and restenosis after coronary angioplasty. Circulation 2003;108:2776-82.
- Cipollone F, Mezzetti A, Porreca E, Di Febbo C, Nutini M, Fazia M, et al. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: effects of statin therapy. Circulation 2002;106:399-402.
- Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation 2002;106:896-9.
- Yan JC, Ma GS, Wu ZG, Li L, Kong XT, Zong RQ, Zhan LZ. Increased levels of CD40-CD40 ligand system in patients with essential hypertension. Clin Chim Acta 2005;355:191-6.
- de Winter RJ, Heyde GS, Koch KT, Fischer J, van Straalen JP, Bax M, et al. The prognostic value of pre-procedural plasma C-reactive protein in patients undergoing elective coronary angioplasty. Eur Heart J 2002;23:960-6.
- Kwaijtaal M, van Diest R, Bar FW, van der Ven AJ, Bruggeman CA, de Baets MH, et al. Inflammatory markers predict late cardiac events in patients who are exhaust after percutaneous coronary intervention. Atherosclerosis 2005;182:341-8.
- Tanne D, Haim M, Goldbourt U, Bovko V, Reshef T, Adler Y, et al. CD40 ligand and risk of ischemic stroke or coronary events in patients with chronic coronary heart disease. Int J Cardiol 2006;107:322-6.
- Turker S, Guneri S, Akdeniz B, Ozcan MA, Baris N, Badak O, et al. Usefulness of preprocedural soluble CD40 ligand for predicting restenosis after percutaneous coronary intervention in patients with stable coronary artery disease. Am J Cardiol 2006;97:198-202.
- Schonbeck U, Libby P. CD40 signaling and plaque instability. Circ Res 2001;89:1092-103.