Effect of TGF-β1 antisense oligodeoxynucleotide on renal function in chronic renal failure rats1
Introduction
A large body of experimental evidence has suggested that transforming growth factor (TGF)-β1 is an important mediator of chronic fibrosis in a range of disease states, including the development of glomerulosclerosis in chronic renal failure (CRF)[1–4]. TGF-β1 is a 25 kDa multifunctional homodimeric polypeptide growth factor with a wide range of biological effects[5,6]. It regulates biological processes, such as cell proliferation, differentiation, and immunological reaction[1,7]. An important function of TGF-β1 is the acceleration of tissue healing by increasing the synthesis of the extracellular matrix and suppressing its degradation by reducing the activity of metalloproteinases and increasing the production of the tissue inhibitors of these proteinases[8].
Studies have shown that the injured glomeruli express more TGF-β1 mRNA, synthesize more TGF-β1 protein, and produce more extracellular matrixes compared to normal glomeruli[9]. Although there are successful trials that have shown that the production of TGF-β1 in glomerulosclerosis models is suppressed by antibodies against TGF-β1, so far, there have been no reports on the assessment of renal function following the blockade of TGF-β1 activity in these models. Furthermore, the use of such antibodies in humans is not feasible because they may cause serum sickness or immune complex formation when administered over prolonged periods of time[10]. Therefore, there is a need for alternative options to be explored. One possible alternative could be the design and use of antisense oligodeoxynucleotides (ODN) to TGF-β1, which have sequences complementary to TGF-β1 mRNA at the translation initiation region. It has been suggested that the TGF-β1 antisense ODN is able to block the abnormal expression of the TGF-β1 gene[11,12] by binding to a selected length of the mRNA, thereby leading to a reduction in TGF-β1 activity. Thus, the present study was designed to investigate the effectiveness of the TGF-β1 antisense ODN in ameliorating the deterioration of kidney function in a rat model of CRF based on a functional and morphological evaluation.
Materials and methods
General preparations All of the experiments were approved by the local Animal Ethical Care Committee and conformed to national guidelines. Male Wistar Kyoto rats (150–220 g) were unilaterally nephrectomized 3 weeks prior to the commencement of the study. Four groups were prepared as follows: Group A (control group; n=14) received a subcutaneous (sc) injection of saline (1 mL/kg) for 8 weeks; Group B (n=19), C (n=13), and D (n=8) received sc injections of puromycin (20 mg/kg) for the same period of time to induce CRF[13]. The TGF-β1 antisense ODN (Alta Bioscience, University of Birmingham, UK) and scrambled ODN (1 mg/kg, intravenously administered; diluted in glucose) was given to rats in groups C and D, respectively. In these rats, the TGF-β1 antisense ODN and the scrambled ODN was given 24 h after the 6th, 7th, and 8th puromycin injections. Puromycin was given as a sc injection while the ODN were given intravenously via the tail vein of the animals that were briefly anaesthetized with a halothane/O2 mixture. The sequence of the TGF-β1 antisense ODN was 5´-CGAGGGCGGCATGGG-3´ while the sequence of the scrambled ODN was 5´-GCATGGGCGAGGGGC-3´[14]. Two other groups (groups A and B) were given glucose intravenously instead of ODN. At the end of the 8 week period, the rats were anaesthetized with pentobarbitone sodium (60 mg/kg, intraperitoneally administered) for the acute study.
Acute study After tracheostomy, the left jugular vein and carotid artery were cannulated for continuous infusion of anesthesia (12.5 mg·kg–1·h–1 at 3 mL/h in 140 mmol/L NaCl) and the measurement of blood pressure, respectively[15–17]. The left femoral artery was cannulated for blood collection. The left kidney was exposed and the ureter was cannulated for urine collection. An electromagnetic flow probe (Carolina square wave electromagnetic flowmeter and EP 100 series probe; Carolina Medical, NC, USA) was fitted around the renal artery for renal blood flow measurement. Upon stabilization, 4 30 min clearances were taken. Arterial blood samples were taken at the beginning and at the end of every 2 clearances. At the end of the acute study, the animals were killed by a rapid intravenous injection of 1 mL sodium pentobarbital, and the kidney was collected for the morphological study.
Chemical analysis The urine protein concentration was measured using a bicinchoninic acid protein assay kit (Bio-Rad, USA). Inulin clearance was calculated from urine and plasma inulin concentrations, measured as previously described[19], and used as a measure of glomerular filtration rate. Urine and plasma electrolytes were measured by flame photometry. The expression of TGF-β1 in the kidney of the rats was analyzed by measuring TGF-β1 protein levels in the kidney cortex and medulla using an ELISA-based method (TGF-β1 BD OptEIA ELISA; Pharmingen, USA)[20].
Morphological study After fixing in formalin for at least 24 h, the kidney tissues were processed using an automatic tissue processor (Leica, Germany). Paraffin blocks with individual kidney tissue embedded in each block were produced. Thin 4–5 mm kidney tissue sections were obtained from the paraffin blocks and stained with hematoxylin-eosin. All of the slides were examined under a light microscope with the help of a certified pathologist who was unaware of the experiment protocols applied to avoid any bias.
Statistical analysis Data were expressed as mean±SEM and were analyzed using two-way ANOVA followed by Bonferroni/Dunn (all means) post-hoc test (Superanova; Abacas, Berkeley, CA, USA). Experimental differences were considered significant if P<0.05.
Results
There was a gain in body weight in all groups throughout the entire 8 week study. However, all the CRF rats, including those treated with either the antisense or scrambled ODN, showed a slight decline in body weight toward the end of the experimental period. The weight of the animals at the end of the 8 week period and at the start of the acute study is given in Table 1. Blood pressure at the start of the experimental protocol was similar in all experimental groups and is shown in Table 1.
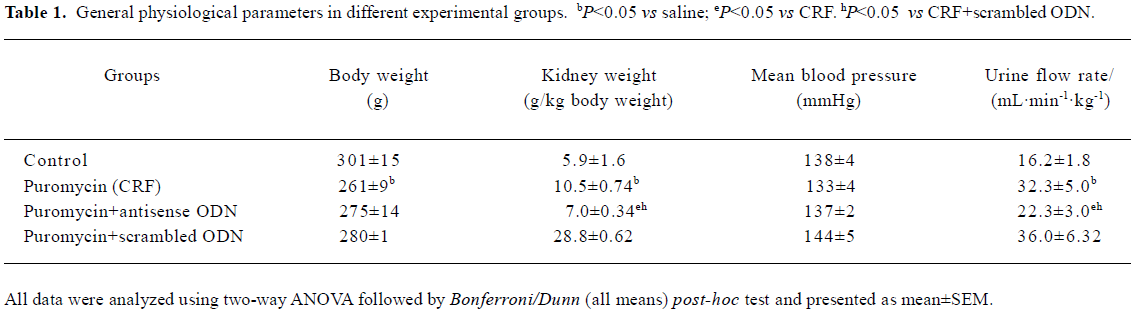
Full table
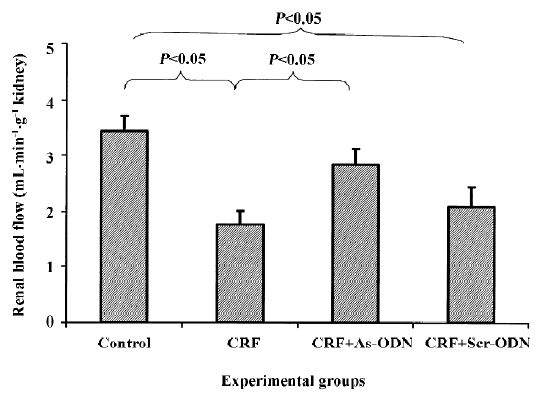
Renal hemodynamic function Renal blood flow was significantly lower in the CRF group, approximately 50% compared to the control group (1.76±0.26 vs 3.44±0.27 mL· min-1·g-1 kidney weight; P<0.05; Figure 1), but decreased by only 17% in the CRF rats treated with the TGF-β1 antisense ODN (2.09±0.36 mL·min–1· g–1 kidney weight, P<0.05). By contrast, the renal blood flow in the group treated with scrambled ODN was also lower to a degree compared to the untreated CRF group at 1.76±0.26 mL·min-1·g-1 kidney weight (Figure 1).
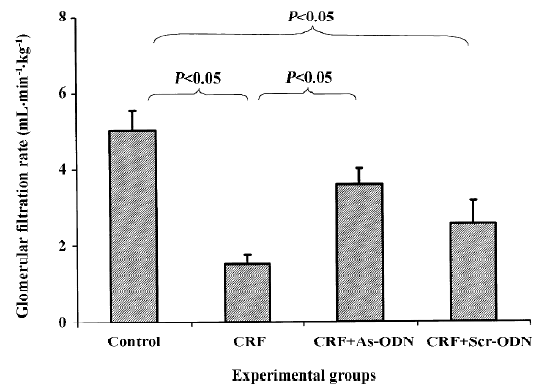
It is evident that the glomerular filtration rate (GFR) in the CRF group was only 30% of that of the saline-treated animals (1.52±0.24 vs 5.02±0.53 mL·min–1·kg–1, P<0.05). However, following the TGF-β1 antisense ODN treatment, the GFR was only 29% lower than that of the saline control group (3.55±0.43 mL·min–1·kg–1, P<0.05). In contrast, the scrambled ODN treatment did not have any significant effect, so the GFR levels were not different from those of the CRF rats (2.54±0.59 mL· min–1· kg–1, Figure 2).
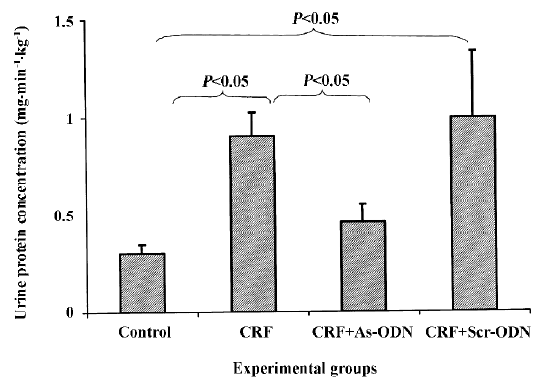
Renal excretory function The urine flow rate was markedly lower in the control rats as compared to that in the CRF and CRF-scrambled ODN-treated groups (P<0.05; Table 1). Urinary protein excretion in the acute study was approximately 3-fold higher in the CRF rats compared to the control group (0.90±0.12 vs 0.30±0.05 mg·min–1·kg–1, P<0.05; Figure 3). Following the TGF-β1 antisense ODN treatment, the increase in urinary protein excretion in the CRF rats was attenuated (0.46±0.09 mg·min–1·kg–1, P<0.05), but in the CRF group treated with the scrambled ODN, urinary protein excretion was comparable to the CRF rats at 0.99±0.34 mg·min–1·kg–1.
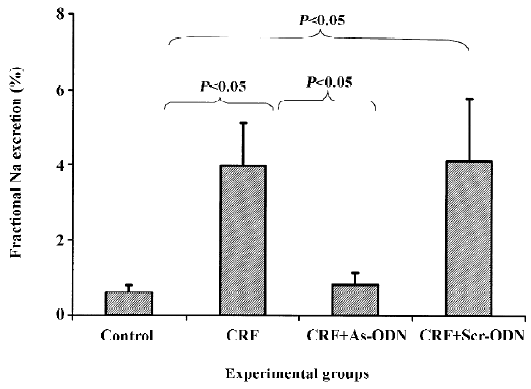
There was no difference in absolute Na+ excretion rates in any of the groups in the study. Interestingly, fractional Na excretion was as much as 6-fold higher in the CRF rats as compared to the control group (3.95±1.15 vs 0.61%±0.18%; P<0.05). This increment was attenuated by the TGF-β1 antisense ODN (0.82%±0.33%, P<0.05), but not by the scrambled ODN (4.09%±1.69%) treatment (Figure 4).
Kidney weight The CRF rats had a 78% higher kidney weight compared to that of the control rats. The kidney weight of the CRF rats treated with the TGF-β1 antisense ODN was only 19% more than the controls and it was also significantly lower than the untreated CRF rats (all P<0.05; Table 1). The kidney weight of the CRF rats treated with the scrambled ODN was not significantly different from that of the untreated CRF rats (P>0.05).
Morphological study Focal segmental glomerulosclerosis (FSGS) was observed in the kidneys of both the untreated and scrambled ODN-treated CRF groups (Figure 5). Periglomerular fibrosis and occasionally pinkish hyalinization around the glomeruli were also observed in the kidneys from these 2 groups. However, these changes around the glomeruli were not seen in the kidneys of the TGF-β1 antisense ODN-treated group.
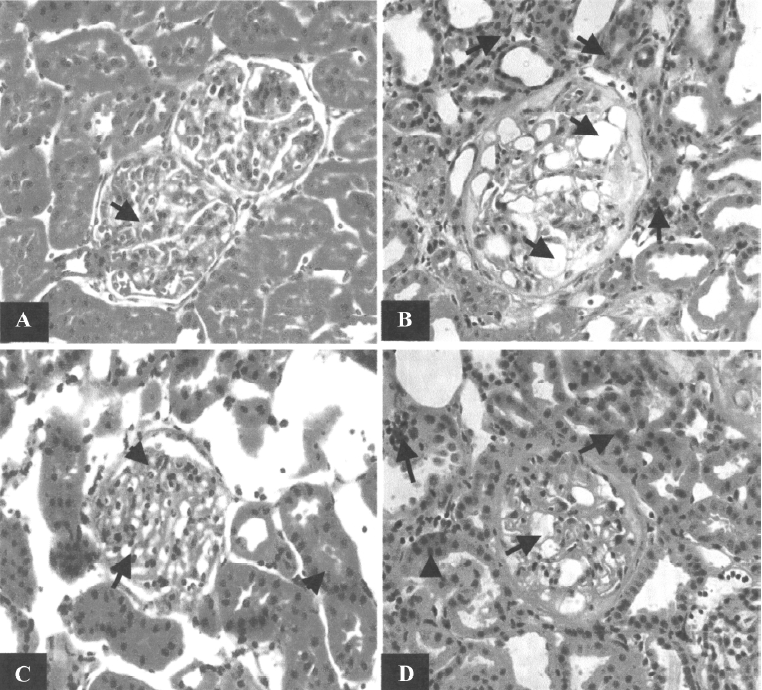
The light microscopic examination also showed that there was a loss of kidney architecture in the kidneys from the untreated and scrambled ODN-treated CRF groups. The histology of the kidneys obtained from these groups showed infiltration of a number of acute inflammatory cells (neutrophils) within the glomerular tufts and chronic inflammatory cells (lymphocytes) in the interstitium (data not shown). Dilatation of tubules in the focal area and some blood vessels with thick walls were also observed. In contrast, in the CRF rats treated with TGF-β1 antisense ODN, even though there was some loss in the architecture of the kidney, the tissue still resembled that of a normal rat. The infiltration of neutrophils in the glomerular tuft was still present and the interstitium showed mild and scattered infiltrate of chronic inflammatory cells predominantly lymphocytes. However, the overall changes were less severe in nature compared to the untreated and scrambled ODN-treated groups (Figure 5).
Expression of TGF-β1 protein levels in the kidney cortex and medulla It was found that the TGF-β1 protein levels in the kidney cortex lysates were not significantly different between the puromycin-induced CRF rats, either untreated (2.567±0.278 pg/μg protein) or treated with the TGF-β1 antisense (2.404±0.264 pg/μg protein) or scrambled ODN (2.155±0.218 pg/μg protein; all P>0.05). Similarly, no significant difference was detected in the lysates of the kidney medulla amongst all these groups: untreated puromycin-induced CRF (2.980±0.336 pg/μg protein), puromycin-induced CRF treated with TGF-β1 antisense ODN (3.286±0.283 pg/μg protein), and puromycin-induced CRF treated with scrambled ODN (3.495±0.410 pg/μg protein; all P>0.05).
Discussion
In the present study, puromycin was used to induce chronic renal failure in rats. Even though the pathogenesis of FSGS following administration of puromycin remains unclear, it has been reported that the level of TGF-β1 was increased in this model of CRF[18]. It has been suggested that TGF-β1 could be one of the mediators which plays a major role in the process of kidney repair following injury. Thus, repeated tissue injury (consequent to repeated dosing with puromycin) could lead to the continuous production of TGF-β1, a progressive deposition of extracellular matrixes, which would eventually result in tissue fibrosis. This abnormal continuous production of TGF-β1 production would convert the repair process into a form of CRF. Therefore, a strategy was developed in an attempt to suppress the overproduction of TGF-β1 by administering the TGF-β1 antisense ODN in order to block the translation of TGF-β1 mRNA and thus reducing the deleterious action of TGF-β1.
An interesting observation of this study was that, in the CRF rats, in spite of obvious changes in the renal functional parameters to the treatment with TGF-β1 ODN, there was no change in the level of TGF-β1 in the kidney tissues. It is important to point out that we chose to utilize a particular dosing regimen, that is, 1 dose of antisense ODN per week, and this was done 24 h after the puromycin challenge. It may well be a possibility that a more effective suppression of the puromycin-induced renal functional and morphological changes could be achieved using different dosing protocols and such a different dosing protocol may accompany a possible change in the level of TGF-β1 in the kidney tissues. Nonetheless, the results derived clearly showed that there might have suppression in the level of TGF-β1, perhaps too small to be detected. However, the relationship between the effect of the puromycin damage and the ability of the TGF-β1 antisense ODN to suppress these responses was very clear, but would need to be explored further with some other dose regimen of the TGF-β1 antisense ODN.
Our observations showed that the weight of the kidneys from CRF rats was higher than that of the control rats (Table 1). This is consistent with the overproduction of extracellular matrixes induced by the continuous production of TGF-β1 in response to the repetitive injury caused by puromycin. Indeed, TGF-β1 has been reported to be a prime candidate in mediating the development of renal hypertrophy[10]. Even though TGF-β1 is typically known to be an inhibitor of cell growth, it actually prevents only the synthesis of DNA and cell proliferation, but promotes mRNA and protein synthesis, therefore enhancing cellular hypertrophy[10]. The treatment of the CRF rats with the TGF-β1 antisense ODN to a large degree prevented the hypertrophy of the kidneys, as reflected by the attenuation of the increased kidney weight compared to the untreated CRF group (Table 1). One possibility is that this might be due to the suppression of the extracellular matrix accumulation in the kidney following the TGF-β1 antisense ODN treatment, thus decreasing the cellular hypertrophy. Our findings are supported by an earlier reported finding in which it is stated that the TGF-β1 antisense ODN administration could reduce the kidney weight in diabetic mice with kidney hypertrophy[21].
The occurrence of proteinuria in the CRF rats is likely due to the damage of the glomerular basement membrane and the epithelial cells elicited by the puromycin administration; this is also a feature of chronic renal failure. Under such circumstances, to promote the wound-healing process, TGF-β1 production would induce the deposition of extracellular matrixes and also their own production. However, repeated puromycin challenges would lead to continuous injury of the tissue and a positive feedback mechanism whereby a vicious circle of TGF-β1 stimulation is created. This would result in an overproduction of extracellular matrix and fibrotic tissue, thereby preventing the normal repair mechanism of the glomerular basement membrane and epithelial cells to take place adequately. The level of proteinuria was reduced in the CRF rats treated with the TGF-β1 antisense ODN (Figure 3). This observation would suggest that in the presence of the TGF-β1 antisense ODN, the overproduction of TGF-β1 was probably blunted, resulting in decreased extracellular matrix and fibrotic tissue accumulation, thus enabling the repair mechanism of the glomerular basement membrane and epithelial cells to adequately take place.
The reduction in renal blood flow in the CRF group may indicate that an increase in intrarenal arterial resistance has occurred. The treatment of CRF rats with the TGF-β1 antisense ODN prevented the reduction in renal blood flow (Figure 1), suggesting that the resistance of the renal vasculature was lower in the TGF-β1 antisense ODN-treated group as compared to the untreated group. Another finding from our study was that the CRF rats had a lower GFR compared to the control group (Figure 2). This might be due to fibrosis developing within the glomeruli leading to a decrease in the glomerular surface area, thereby resulting in a fall in ultrafiltration coefficient, Kf[19]. The treatment of the CRF rats with the TGF-β1 antisense ODN attenuated the percentage reduction of GFR in the CRF rats consistent with the argument that the TGF-β1 antisense ODN tends to suppress the vicious cycle of TGF-β1 and fibrotic tissue overproduction. This suggestion is supported by the observation that no glomerulosclerosis were observed in the kidneys from the CRF rats treated with the TGF-β1 antisense ODN (Figure 5).
The fractional Na+ excretion was higher in the CRF group compared to the control group (Figure 4), probably as a consequence of the decreased reabsorptive ability of the tubules. This could have resulted from the continuous damage of the tubules caused by repeated insults from the puromycin challenges related to the abnormal repair of the tubular cells. The magnitude of rise in fractional Na+ excretion in the CRF rats was significantly attenuated following treatment with the TGF-β1 antisense ODN. This observation may suggest that the tubules were adequately repaired in the presence of the TGF-β1 antisense ODN, thereby enabling the reabsorption of Na+ to take place appropriately.
Regarding the effects TGF-β1 antisense on the tubular function of the CRF rats, a similar conclusion that the impaired tubular reabsorptive ability caused by puromycin-induced nephrotoxic insult, ameliorated by the antisense treatment, came from the data obtained from the urine flow rate. It was observed that in the CRF rats, puromycin caused marked diuresis, which was brought almost to a normal level by treatment with the TGF-β1 antisense ODN.
In conclusion, the administration of the TGF-β1 antisense ODN to CRF rats could reduce the severity of proteinuria and improve the renal blood flow as well as GFR and plasma inulin concentration. It also improved the reabsorptive function of tubules, as reflected by the attenuation of the raised fractional Na+ excretion. Moreover, the increased kidney weight in the CRF rats was partly prevented following the TGF-β1 antisense ODN treatment. These improvements in the kidney function of the CRF rats following the TGF-β1 antisense ODN treatment could be due to the suppression of extracellular matrix and fibrotic tissue overproduction, as demonstrated by the light microscopic examination of the kidneys. Most importantly, the TGF-β1 antisense ODN brought all these changes virtually without the expression of renal TGF-β1. It is important to note that with a single weekly dose, the TGF-β1 antisense ODN has very strong ameliorative activity in correcting the compromised renal function and morphological changes in puromycin-induced CRF rats.
Taken together, these findings suggest that the TGF-β1 antisense ODN has the potential to ameliorate the severity of the deterioration of kidney function in rats with CRF induced by repeated puromycin administration. The results also indicate that the TGF-β1 antisense ODN in part prevented the inappropriate repair mechanism following puromycin-induced injury to progress to a state of chronic fibrotic disease, resulting in improved renal function.
Acknowledgements
We would like to thank the Departments of Pharmacology and Allied Health Science, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia for the provision of their facilities.
References
- Border WA, Noble NA. Transforming growth factor-β in tissue fibrosis. N Engl J Med1 1994; 10: 1286–92.
- Creely JJ, DiMari SJ, Howe AM, Haralson MA. Effects of transforming growth factor-β on collagen synthesis by normal rat kidney epithelial cells. Am J Pathol 1992;140:45-55.
- Michael Z, Frank S, Gerhard AM. Renal fibrosis: an update. Curr Opin Nephrol Hypertens 2001;10:315-20.
- O’Shea M, Miller SB, Finkel K, Hammerman MR. Roles of growth hormone and growth factors in the pathogenesis and treatment of kidney disease. Curr Opin Nephrol Hypertens 1993;2:67-72.
- Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor β. Biochim Biophys Acta 1990;1032:79-87.
- Lyons RM, Moses HL. Transforming growth factors and the regulation of cell proliferation. Eur J Biochem 1990;187:467-73.
- Roberts AB, Flanders KC, Heine UI, Jakowlew S, Kondaiah P, Kim SJ, et al. Transforming growth factor-β: multifunctional regulator of differentiation and development. Phil Trans R Soc Lond B 1990;327:145-54.
- Engelmeyer E, Van Goor H, Edwards DR, Diamond J. Differential mRNA expression of renal cortical tissue inhibits metallo-proteinases 1, 2 & 3 in experimental hydronephrosis. J Am Soc Nephrol 1995;5:1675-83.
- Okuda S, Languino LR, Ruoslahti E, Border WA. Elevated expression of transforming growth factor β and proteoglycan production in experimental glomerulonephritis. J Clin Invest 1990;86:453-62.
- Sharma K, Ziyadeh FN. Emerging role of transforming growth factor-β in kidney diseases. Am J Physiol 1994;266:F829-42.
- Askari FK, McDonnell WM. Antisense-oligonucleotide therapy. N Engl J Med 1996;334:316-8.
- Neckers L, Whitesell L. Antisense technology: biological utility and practical consideration. Am J Physiol 1993;265:L1-12.
- Saito T, Sumithran E, Glasgow EF, Atkins RC. The enhancement of aminonucleoside nephrosis by the co-administration of protamine. Kidney Int 1987;32:691-9.
- Akagi Y, Isaka Y, Arai M, Kaneko T, Tenaka M. Inhibition of TGF-β1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 1996;50:148-55.
- Armenia A, Munavvar AS, Abdullah NA, Helmi A, Johns EJ. The contribution of adrenoceptor subtype(s) in the renal vasculature of diabetic spontaneously hypertensive rats. Br J Pharmacol 2004;142:719-26.
- Hye Khan MA, Sattar MA, Abdullah NA, Johns EJ. Cisplatin cause altered renal hemodynamics in rats: role of augmented a-adrenergic responsiveness. Exp Toxicol Pathol 2007;59:253-60.
- Khan AH, Sattar MA, Abdullah NA, Johns EJ. Influence of cisplatin-induced renal failure on the alpha (1)-adrenoceptor subtype causing vasoconstriction in the kidney of the rat. Eur J Pharmacol 2007;569:110-8.
- Tofovic SP, Dubey R, Salah EM, Jackson EK. 2-Hydroxyestradiol attenuates renal disease in chronic puromycin aminonucleoside nephropathy. J Am Soc Nephrol 2002;13:2737-47.
- Hestin D, Johns EJ. The influence of allopurinol on kidney haemodynamic and excretory responses to renal ischaemia in anaesthetised rats. Br J Pharmacol 1999;128:255-61.
- Yao Q, Qian JQ, Lin XH, Lindholm B. Inhibition of the effect of high glucose on the expression of Smad in human peritoneal mesothelial cells. Int J Artif Organs 2004;27:828-34.
- Han DC, Hoffman BB, Hong SW, Guo J, Ziyadeh FN. Therapy with antisense TGF-β1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am J Physiol (Renal Physiol) 2000;278:F628-34.
