Protective effects of heat shock protein70 induced by geranyl-geranylacetone in atrophic gastritis in rats1
Introduction
Gastric carcinoma remains one of the most prevalent malignant diseases in the world[1]. As there is no efficient method for early diagnosis and therapy, the 5-year survival rate for most patients with advanced gastric cancer is less than 20%[2]. Atrophic gastritis is well known as a premalignant disease with an increased risk in developing into gastric carcinoma[3]. Therefore, several gastroenterologists have emphasized the importance of intervention in atrophic gastritis for the prevention of gastric cancer[4].
Atrophic gastritis results from the long-term gastric damages that impair the adaptive cytoprotection of gastric mucosa. In recent years, the mechanism that protects the gastric mucosa against intrinsic and extrinsic stimuli and maintains the proper structure and function of the gastric mucosa has attracted considerable attention[5]. Heat shock protein (HSP)70, one of the major molecular chaperons, can adapt cells to cope with various stresses in gastric mucosa[6] and accelerate the cellular recovery from different stimuli by coping with unfolded or denatured proteins[7]. The overexpression of HSP70 has made cells resistant to death by increasing mucosal blood flow in gastric mucosa[8,9].
Geranylgeranylacetone (6,10,14,18-tetramethyl-5,9,13,17-nonadecatetraen-2-one, GGA; Eisai, Japan), an anti-ulcer drug, protects gastric epithelial cells from damage[10,11]. GGA has been shown to increase the protein level of HSP70 in cultured guinea pig gastric mucosal cells and rat gastric mucosa while preventing ethanol-induced gastric damage[12]. In addition, the induction of HSP70 by GGA has found cytoprotective action in a number of pathological lesions, including heart ischemia[13], hepatectomy[14], cerebral infarction[15], and colitis[16]. However, there is no literature regarding the protective effect of GGA on atrophic gastritis. In this study, we investigated whether GGA exerts its protective role in the progression of atrophic gastritis via the induction of HSP70.
Materials and methods
Induction of atrophic gastritis in rats and treatment with GGA Male Sprague-Dawley rats (200–300 g, Grade I) were obtained from the Animal Center of Zhejiang Academy of Medicine (Hangzhou, China). The study was in compliance with the Declaration of Helsinki. The rats were maintained on a 12 h light/12 h dark cycle with food and water ad libitum. Atrophic gastritis in rats was induced as reported previously[17]. In brief, the rats were administered 0.1% ammonia solution, 60% ethanol (1 mL/kg, ig on every Tuesday and Friday) and 20 mmol/L deoxycholic acid (1 mL/kg, ig every day) for 24 weeks. Accompanied by the induction of atrophic gastritis, the rats (n=6) were treated with GGA (200 mg/kg, ig) for 8 weeks, from week 17 to the end of week 24. To further investigate whether GGA protected gastric mucosa via the induction of HSP70, we administered quercetin (100 mg/kg ig, n=6; Sigma, Saint Louis, MO, USA), an inhibitor of HSP70 synthesis[18–20], in DMSO to rats daily for the final 8 weeks of the 24-week period of atrophic gastritis induction. The control rats were gavaged with DMSO (5 mL/kg, n=6). All the rats were then sacrificed and the gastric tissues in lesser curvatures were excised for further analyses. One half of each sample (within 1 cm from the pylorus) was immediately frozen in liquid nitrogen and stored at -80 °C for the Western blot analysis. The other half was fixed in 4% buffered formalin for histological analysis and immunohistochemistry.
Inflammation index scoring in the antrum The sections were stained with HE. A histological evaluation of the severity of inflammation was performed by a scoring criterion in accordance with the Sydney system[21]. The inflammation score was assigned based on the following scale: 0=normal; 1=minimal inflammatory cells in pit or basal region of gastric mucosa; 2=moderate number of inflammatory cells mainly in two thirds of gastric mucosa; and 3=a large number of inflammatory cells in gastric mucosa. Five random fields of vision in the antrum were chosen in each section.
Quantitative analysis of gastric mucosal thickness and gastric glands in the antrum Gastric mucosal thickness and the quantity of gastric glands of 1 mm horizontal length were measured with a micrometer eyepiece (Olympus Optical, Tokyo, Japan) on HE-stained sections from the gastric samples. Five random fields of vision in the antrum were chosen in each section.
Immunohistochemistry evaluation The distribution of HSP70 in gastric mucosa was assessed with immuno-histo-chemistry. Tissues from rats were sectioned at 5 μm and mounted on poly L lysine coated slides. Antigen retrieval was carried out by boiling sections in 10 mmol/L citrate buffer (pH 6.0) for 10 min, and endogenous peroxidase activity was blocked in methanol containing 3% hydrogen peroxide for 15 min. After blocking with 5% normal rabbit serum in phosphate buffered solution (PBS) for 20 min, the sections were incubated with mouse monoclonal antibody against human HSP70 (1:1000, Sigma, USA) at 4 °C overnight. Non-specific mouse immunoglobulin G (IgG) was applied as the negative control. After washing in PBS, the sections reacted with the biotinylated rabbit anti mouse IgG for 15 min at room temperature and with the peroxidase-labeled streptavidin for another 15 min. Then sections were stained with diamino-benzidine-H2O2 solution for 3 min and counterstained with hematoxylin.
Western blot analyses The total proteins isolated from the gastric tissues were resolved by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking in 5% non-fat, dry milk, the membranes were subjected with anti-HSP70 (1:6000, Sigma, USA) primary antibody at 4 °C overnight and then washed 3 times. The membranes were then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biote-chnology, Santa Cruz, CA, USA) at a 1:5000 dilution for 1 h at room temperature. The blotted membrane was visualized by chemiluminescent substrate (EZ-ECL, Kibbutz Beit Haemek, Israel). The immunoblotting for β-actin (1:1000, Santa Cruz Biotechnology, USA) was used as a loading control, and the densitometry was performed on Image-Pro Plus 5.0 software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis Data were expressed as median values. Differences between groups were examined for statistical significance using non-parametric tests (median test). P<0.05 denoted the presence of a statistical difference.
Results
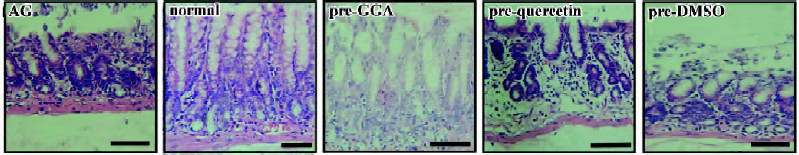
Pathological changes in rats with atrophic gastritis After 24 weeks of administration with ammonia solution, ethanol, and deoxycholic acid, the rats developed atrophic gastritis with an infiltration of lymphocytes into gastric mucosa and loss of glandular cells in the gastric antrum (Figure 1). A quantitative analysis showed a significant increase of the inflammation index in atrophic gastritis compared to that in the controls (P<0.05; Table 1). The thickness of gastric mucosa and the quantity of gastric glands were obviously reduced in the rats with induced atrophic gastritis (vs controls, P<0.05; Table 1).

Full table
Effect of GGA on the progression of atrophic gastritis in rats Concomitant with the induction of atrophic gastritis in rats, we administered GGA for 8 weeks and found that GGA protected gastric mucosa from the continuous damages and improved the pathological changes in gastric mucosa compared with the atrophic gastritis group (Figure 1). GGA decreased the inflammation index and significantly increased the gastric mucosal thickness and glandular quantity (vs atrophic gastritis, P<0.05; (Table 1). On the other hand, quercetin accelerated the progression of atrophic gastritis in the rats by significantly increasing the inflammatory cells in the gastric antrum (vs vehicle control, P<0.05) and aggravating the loss of glandular cells (Table 1).
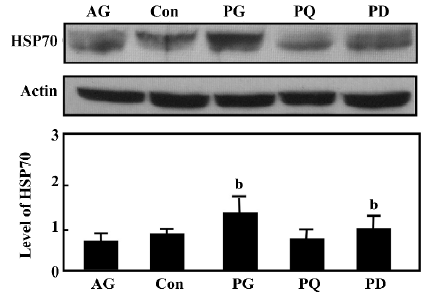
Level of HSP70 expression in the gastric antrum of rats Immunoblotting revealed a reduction of HSP70 expression in the gastric antrum of rats with atrophic gastritis. Treatment with 200 mg/kg GGA for 8 weeks significantly increased the accumulation of HSP70, which was nearly 2-fold higher than that in atrophic gastritis of rats (P<0.05). In contrast, quercetin decreased the level of HSP70 in the gastric mucosa compared with the vehicle-treated rats (Figure 2).
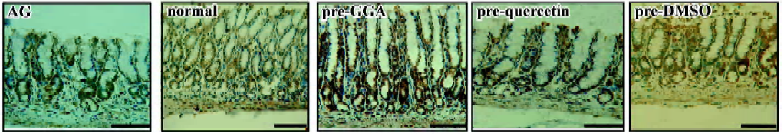
Distribution of HSP70 expression in the gastric antrum HSP70 immunostaining was detectable in all gastric samples from the rats by immunohistochemistry analyses. Cytoplasmic staining was more frequent in the gastric epithelial cells at the crest of mucosal folds. Conversely, nuclear staining was mainly localized to the cells at the base of mucosal folds. In the rats with atrophic gastritis, the nuclear immunoreactivity of HSP70 was observed. The staining of HSP70 in the GGA-treated rats was distributed in the whole gastric mucosa, in both the nucleus and cytoplasm, but in the quercetin-treated rats, HSP70 was mainly recognized in the cytoplasm of the cells at crest of gastric mucosal folds, which was less frequent than that in the vehicle control (Figure 3).
Discussion
Atrophic gastritis is characterized with chronic inflammation of the gastric mucosa and loss of gastric glandular cells with replacement by intestinal-type epithelium, pyloric-type glands, and fibrous tissue[22]. Damage to the gastric mucosa has been reported to correlate with the etiology of atrophic gastritis. Helicobacter pylori (H pylori) infection is by far the most common cause of chronic atrophic gastritis[23]. The inoculation of H pylori in the gastric antrum caused atrophic gastritis in the Japanese monkey model after 6 months of infection[24]. Epidemiology also revealed a 4.2-fold greater odds of atrophic gastritis for H pylori-positive patients than H pylori-negative patients[25]. H pylori produces ammonia in the stomach by the hydrolysis of urea, which has an etiological role in H pylori-associated atrophic gastritis[26]. In addition, bile reflux and alcohol consumption could be potential risk factors for gastric atrophy and intestinal metaplasia[25,27]. In this study, we integrated the multiple factors including H pylori, bile, and ethanol to induce atrophic gastritis in rats. As bile reflux diminished H pylori from gastric mucosa[28], ammonia solution instead of live H pylori was employed to simulate the conditions of H pylori infection, and atrophic gastritis was successfully induced, which showed the significant infiltration of inflammatory cells and loss of glands in gastric mucosa. Moreover, our previous study reported that the combined administration of 60% ethanol, 20 mmol/L deoxycholic acid, and 0.5 g/L ammonia for 12 weeks could induce gastritis with early features of glandular atrophy in rats[17]. After 16 weeks of inducing treatments, atrophic gastritis came into being in rats with notable chronic inflammation and the loss of gastric glandular cells. Further progression of atrophic gastritis was observed after treatment for 24 weeks. Accordingly, GGA was administered at week 17 when atrophic gastritis was just induced and continued into week 24 when we investigated the effect of GGA on the progression of atrophic gastritis.
In recent years, GGA has been reported to exert a protective role in a variety of animal models[12,16]. In mice with trinitrobenzene sulfonic acid-induced colitis, the administration of GGA by oral gavage at 300 mg/kg suppressed inflammation in the colons, and significantly improved mouse survival rate[16]. GGA plays a cytoprotective role against acute gastric mucosal lesions induced by chemicals[29,30]. It has also been shown that GGA promotes the healing of acetic acid-induced chronic gastric ulcers in rats[31]. In this study, we demonstrated for the first time that the administration of GGA in rats with atrophic gastritis results in protection against further progression of atrophic gastritis. GGA diminished the continuous damage to gastric mucosa and facilitated histological recovery with inflammation relief and glandular restoration.
One of the defense mechanisms of GGA has been clarified to increase the expression of HSP70 to protect cells against stresses. HSP70 is an important endogenous cytopro-tective factor. GGA increased the protein level of HSP70 in gastric mucosa of rats while preventing ethanol-induced gastric damage[12]. The induction of HSP70 expression is beneficial for preventing intestinal atrophy and maintaining mucosal functions in intestinal cells[32]. Gastric atrophy results from the long-term damage to gastric mucosa, and an aberrant apoptosis is suggested to be involved in its pathogenesis[33]. HSP70 is tightly related with the stability of cells to damage by interference with apoptotic programs[6,9]. In our study, the 2-fold level of HSP70 was induced by GGA in both the cytoplasm and nucleus of gastric cells. HSP70 accumulation could accelerate the recovery of atrophic gastritis via modulating apoptosis and restoring the damaged cellular structures[34,35]. Moreover, Pierzchaiski has indicated that H pylori decreases the synthesis of HSP70 in gastric epithelial cells by the inactivation of heat shock factor 1 in the recent study[36]. The inhibition of HSP70 disturbed gastric adaptation and facilitated H pylori to avoid host immunity[37]. H pylori eradication, which has been reported to reverse the progression of atrophic gastritis[38], is accompanied by increased HSP70 expression[39]. The induction of HSP70 by GGA might interrupt the damage from H pylori. Additional studies are needed to evaluate this relationship.
Quercetin is known to block the synthesis of HSP70 at the level of mRNA accumulation[19] and then eliminates the protective role of HSP70 to interrupt cell recovery from damage[20,21]. Our data showed that quercetin suppressed the accumulation of HSP70 in gastric cells, especially in the nucleus, and increased the infiltration of inflammatory cells in gastric mucosa. HSP70 depletion could aggravate inflammation by significantly increasing the activation of NF-κB and other inflammatory cytokines[40]. The suppression of HSP70 was supposed to correlate with the progression of atrophic gastritis, which further supported our findings that HSP70 induced by GGA protected gastric mucosa from continuous damage and facilitated the recovery of atrophic gastritis in rats.
In conclusion, GGA prevents the progression of atrophic gastritis via the induction of HSP70 expression. Therefore, a potential drug target for the treatment of atrophic gastritis is suggested.
Acknowledgement
We sincerely express our gratitude to Mr Yun-bin YAO, Animal Laboratory of Medicine, Sir Run Run Shaw Hospital (Hangzhou, China), for assisting with the animal model.
References
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001;2:533-43.
- Correa P. Is gastric cancer preventable? Gut 2004;53:1217-9.
- Sipponen P. Atrophic gastritis as a premalignant condition. Ann Med 1989;21:287-90.
- Dixon MF. Prospects of intervention in gastric carcinogenesis: reversibility of gastric atrophy and intestinal metaplasia. Gut 2001;49:2-4.
- Kubo N, Noguchi T, Takeno S, Tohara K, Uchida Y, Shimoda H. Injury to the gastric mucosa and cellular dynamics in a rat model of duodenogastric reflux: the possible significance of gastrin induction and a heat shock protein. Surg Today 2000;30:999-1004.
- Rokutan K. Role of heat shock proteins in gastric mucosal protection. J Gastroenterol Hepatol 2000; 15 Suppl[15]: D12–9.
- Basu S, Srivastava PK. Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones 2000;5:443-51.
- Tomisato W, Tsutsumi S, Tsuchiya T, Mizushima T. Geranylgeranylacetone protects guinea pig gastric mucosal cells from gastric stressor-induced necrosis by induction of heat-shock proteins. Biol Pharm Bull 2001;24:887-91.
- Shichijo K, Ihara M, Matsuu M, Ito M, Okummura Y, Sekine I. Overexpression of heat shock protein 70 in stomach of stress-induced gastric ulcer-resistant rats. Dig Dis Sci 2003;48:340-8.
- Ushijima H, Tanaka K, Takeda M, Katsu T, Mima S, Mizushima T. Geranylgeranylactone protects membranes against nonsteroidal anti-inflammatory drugs. Mol Pharmacol 2005;68:1156-61.
- Terano A, Shiga J, Hiraishi H, Ota S, Sugimoto T. Protective action of tetraprenylacetone against ethanol-induced damage in rat gastric mucosa. Digestion 1986;35:182-8.
- Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranyl-acetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology 1996;111:345-57.
- Ooie T, Takahashi N, Saikawa T, Nawata T, Arikawa M, Yamanaka K, et al. Single oral of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation 2001;104:1837-43.
- Oda H, Miyake H, Iwata T, Kusumoto K, Rokutan K, Tashiro S. Geranylgeranylacetone suppresses inflammatory responses and improves survival after massive hepatectomy in rats. J Gastro-intest Surg 2002;6:464-72.
- Uchida S, Fujiki M, Nagai Y, Abe T, Kobayashi H. Geranyl-geranylacetone, a noninvasive heat shock protein inducer, induces protein kinase C and leads to neuroprotection against cerebral infarction in rats. Neurosci Lett 2006;396:220-4.
- Ohkawara T, Nishihira J, Takeda H, Katsurada T, Kato K, Yoshiki T, et al. Protective effect of geranylgeranylacetone on trinitro-benzene sulfonic acid-induced colotis in mice. Int J Mol Med 2006;17:229-34.
- Xiang Z, Si JM, Huang HD. Chronic gastritis rat model and role of inducing factors. World J Gastroenterol 2004;10:3212-4.
- Hosokawa N, Hirayoshi K, Nakai A, Hosokawa Y, Marui N, Yoshida M, et al. Flavonoids inhibit the expression of heat shock proteins. Cell Struct Funct 1990;15:393-401.
- Niu P, Liu L, Gong Z, Tan H, Wang F, Yuan J, et al. Overexpressed heat shock protein 70 protects cells against DNA damage caused by ultraviolet C in a dose-dependent manner. Cell Stress Chaperones 2006;11:162-9.
- Nakada J, Matsura T, Okazaki N, Nishida T, Togawa A, Minami Y, et al. Oral administration of geranylgeranylacetone improves survival rate in a rat endotoxin shock model: administration timing and heat shock protein 70 induction. Shock 2005;24:482-7.
- Dixon MF, Genta RM, Yardley JH. Classification and grading of gastritis. Am J Surg Pathol 1996;20:1161-81.
- Genta RM, Rugge M. Gastric precancerous lesions: heading for an international consensus. Gut 1999;45 Suppl 1:I5-8.
- Ohkuma K, Okada M, Murayama H, Seo M, Maeda K, Kanda M, et al. Association of Helicobacter pylori infection with atrophic gastritis and intestinal metaplasia. J Gastroenterol Hepatol 2000;15:1105-12.
- Kodama M, Murakami K, Sato R, Okimoto T, Nishizono A, Fujioka T. Helicobacter pylori-infected animal models are extremely suitable for the investigation of gastric carcinogenesis. World J Gastroenterol 2005;11:7063-71.
- You WC, Zhang L, Gail MH, Ma JL, Chang YS, Blot WJ, et al. Helicobacter pylori infection, garlic intake and precancerous lesions in a Chinese population at low risk of gastric cancer. Int J Epidemiol 1998;27:941-4.
- Tsujii M, Kawano S, Tsuji S, Ito T, Nagano K, Sasaki Y, et al. Cell kinetics of mucosal atrophy in rat stomach induced by long-term administration of ammonia. Gastroenterology 1993;104:796-801.
- Dixon MF, Mapstone NP, Neville PM, Moayyedi P, Axon AT. Bile reflux gastritis and intestinal metaplasia at the cardia. Gut 2002;51:351-5.
- Abe H, Murakami K, Satoh S, Sato R, Kodama M, Arita T, et al. Influence of bile reflux and Helicobacter pylori infection on gastritis in the remnant gastric mucosa after distal gastrectomy. J Gastroenterol 2005;40:661-3.
- Murakami M, Oketani K, Fujisaki H, Wakabayashi T, Ohgo T. Effects of the antiulcer drug geranylgeranylacetone on asprin-induced gastric ulcers in rats. Jpn J Pharmacol 1982;32:299-306. Japanese..
- Ohta Y, Kobayashi T, Inui K, Yoshino J, Kitagawa A, Nakazawa S. Preventive effect of teprenone on acute gastric mucosal lesion progression in compound 48/80-treated rats. Eur J Pharmacol 2004;487:223-32.
- Kobayashi T, Ohta Y, Yoshino J, Nakazawa S. Teprenone promotes the healing of acetic acid-induced chronic ulcers in rats by inhibiting neutrophil infiltration and lipid peroxidation in ulcerated gastric tissues. Pharmacol Res 2001;43:23-30.
- Chow A, Zhang R. Glutamine reduces heat shock-induced cell death in rat intestinal epithelial cells. J Nutr 1998;128:1296-301.
- Moss SF, Valle J, Abdalla AM, Wang S, Siurala M, Sipponen P. Gastric cellular turnover and the development of atrophy after 31 years of follow-up: a case-control study. Am J Gastroenterol 1999;94:2109-14.
- Garrido C, Gurbuxani S, Ravaqnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun 2001;286:433-42.
- Pelham HRB. HSP70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J 1984;3:3095-100.
- Pierzchaiski P, Krawiec A, Ptak-Belowska A, Barariska A, Konturek SJ, Pawlik WW. The mechanism of heat-shock protein 70 gene expression abolition in gastric epithelium caused by Helicobacter pylori infection. Helicobacter 2006;11:96-104.
- Huff JL, Hansen LM, Solnick JV. Gastric transcription profile of Helicobacter pylori infection in the rhesus macaque. Infect Immun 2004;72:5216-26.
- Arkkila PE, Seppala K, Farkkila MA, Veijola L, Sipponen P. Helicobacter pylori eradication in the healing of atrophic gastritis: a one-year prospective study. Scand J Gastroenterol 2006;41:782-90.
- Konturek JW, Fischer H, Konturek PC, Huber V, Boknik P, Luess H, et al. Heat shock protein 70 (hsp70) in gastric adaptation to aspirin in Helicobacter pylori infection. J Physiol Pharmacol 2001;52:153-64.
- Sinqleton KD, Wischmeyer PE. Effects of HSP70.1/3 gene knockout on acute respiratory distress syndrome and the inflammatory response following sepsis. Am J Physiol Lung Cell Mol Physiol 2006;290:L956-61.
