Resistin-binding peptide antagonizes role of resistin on white adipose tissue1
Introduction
Traditionally, adipose tissue is only considered to be energy storage. When energy is required, free fatty acids (FFA) are released from adipose triglyceride stores into the circulation by lipolysis and oxidization[1]. Recently, adipose tissue has also being recognized as an important endocrine organ with the discoveries of several adipocyte-secreted molecules. Using these molecules, adipocytes are able to communicate with other tissues and organs to regulate lipid and glucose metabolism, energy balance and insulin action[2,3].
Resistin (Retn, NM_022984) is a newly discovered adipo-cyte hormone (adipocytokine) involved in the development of insulin resistance[4]. Several studies have shown resistin as a potential link between obesity and type 2 diabetes mellitus (T2DM)[5,6]. Recently, direct functional effects of resistin on some of insulin target organs were described, such as L6 skeletal muscle cells, 3T3-L1 adipocytes and the liver[7–9].
Resistin-binding peptide (RBP) was coded by 3 cDNA fragments identified by screening a cDNA phage display library of rat multiple tissues. Three cDNA fragments have the same 11 bp 5' sequence. Studies have shown that RBP can bind to resistin specifically. Overexpression of RBP in 3T3-L1 pre-adipocytes can inhibit the stimulatory role of resistin on adipocytes differentiation[10]. All these data suggest that RBP may be the therapeutic blockers of insulin resistance in diabetic patients.
However, no data are currently available on potential direct paracrine or autocrine effects of resistin on adipose tissue, and there are no known reports on the relationship of RBP and resistin. In this study, we examined the effect of rat resistin protein (rResistin) on rat white adipose tissue (WAT) in primary cultures to demonstrate direct actions of resistin on lipid metabolism and adipocytokine expression. In addition, we provide the evidence that RBP administration can antagonize these roles of resistin.
Materials and methods
Adipose tissue culture conditions Epididymal adipose tissue fragments from male Sprague-Dawley (180–220 g) rats were placed in serum-free M199 (Gibco, Grand Island, NY, USA). Minced tissue of known mass (150 mg) was plated with 5 mL medium containing 30 ng/mL rResistin (Alexis, San Diego, CA, USA) or combined with RBP (N-AWIL-C, Sangon, Shanghai, China) with varying concentrations (1×10-12 mol/L, 1×10-10 mol/L, 1×10-8 mol/L) in each 60 mm Petri dish. The dishes were maintained at 37 °C and 5% CO2: 95% O2. Culture medium supernatant and adipose tissue were collected respectively from each dish after 24 h.
Measurement of lipolysis in WAT fat pads Adipose tissue lipolysis was determined using FFA levels released into the culture medium supernatant[11]. FFA in conditioned medium were measured using an available colorimetric kit (Randox laboratories Co, Antrim, UK). Each experiment was repeated 6 times.
Measurement of adipocytokine levels secreted in WAT The levels of TNF-α and adiponectin were measured by ELISA kit. In brief, we dispensed 100 µL of standards and specimens into appropriate wells and dispensed 50 µL of enzyme conjugate reagent into each well. We incubated the wells at 18–25 °C for 90 min, and rinsed and emptied the microtiter wells with washing buffer. After washing 5 times, we removed residual water droplets and dispensed 50 µL of color A and color B reagent into each well. We gently mixed for 5 s and incubated it at 18–25 °C for 15 min. The reaction was stopped by adding 50 µL of stop solution to each well and then gently mixed for 30 s. The optical density was recorded at 450 nm within 20 min. Each experiment was repeated at least 3 times.
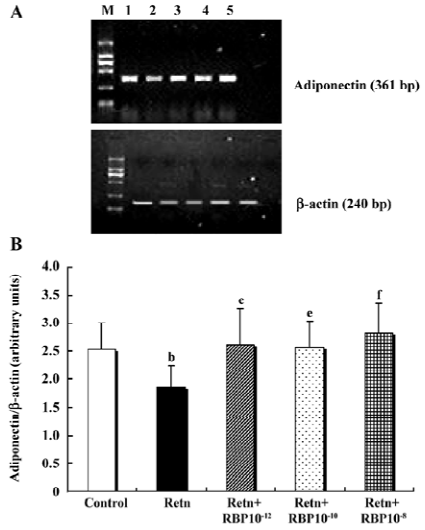
RT-PCR assay for TNF-α and adiponectin RNA was prepared from WAT with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA content was quantified by UV spectrophotometer at 260 nm, and samples were separated by electrophoresis on 1% agarose gels, stained with 0.1 mg/L ethidium bromide and visualized under UV light for confirmation of RNA integrity. Total RNA of 1 µg was reverse transcripted into first-strand complementary DNA (fscDNA) by using an Oligo(dT)15 primer (Promega, Madison, WI, USA). The fscDNA was amplified by PCR. The PCR was carried out in a total volume of 20 µL, containing 20 mmol/ L Tris-HCl, 50 mmol/L KCl, 1.5 mmol/ L MgCl2, 0.2 mmol/ L dNTP (Promega, Madison, WI, USA), 0.6 mmol/L of each primer, and 2.5 units of Ex-Taq DNA polymerase (TaKaRa, Dalian, Liaoning, China). The mRNA expression of the housekeeping gene β-actin was used as an internal standard. Primer sequences and PCR reaction conditions are shown in Table 1. Denaturing, annealing, and extension reactions were performed at 94 ºC for 30 s, at Ta ºC for 30 s, and at 72 ºC for 1 min. The PCR pro-ducts were analyzed on 1.5% agarose gel and stained with ethidium bromide. The levels of gene mRNA were expressed as the ratio of the gene to β-actin.
Full table
Statistical analysis All data are expressed as mean±SEM. Data were analyzed using one-way ANOVA of the SPSS 10.0 statistic software package (Spss, Chicago, IL, USA). The threshold of significance was defined as P<0.05.
Results
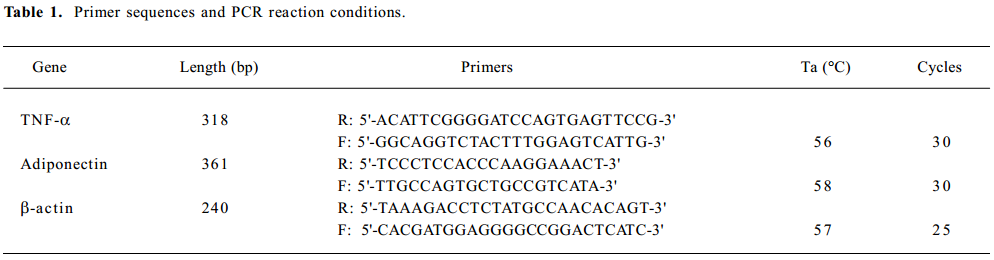
rResistin and RBP regulates lipolysis in WAT fat pads ex vivo Lipolysis of adipose tissue was determined using FFA released in the culture medium supernatant. As shown in Figure 1A, rResistin increase FFA release in fat pads from Sprague-Dawley rats after 24 h, where resistin was a strong stimulation of lipolysis. To ascertain whether this effect was antagonized or enhanced by RBP, a similar experiment was performed combined with varying concentrations of RBP. As shown in Figure 1B, FFA released in the fat pads decreased after RBP was added as expected, whereas there was no difference between the 3 concentrations of RBP (P>0.05).
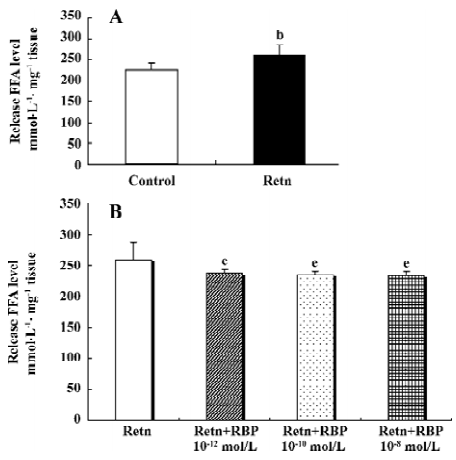
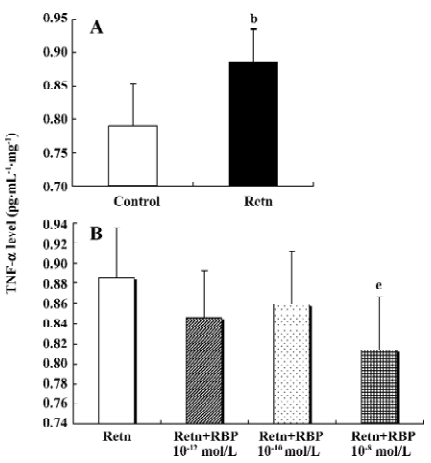
Effects of rResistin and RBP treatment on TNF-α expression in WAT It has been demonstrated that TNF-α is an important pro-inflammatory adipocytokine and can lead to insulin resistance[12]. Studies have found that it activates resistin mRNA expression in vitro[13]. To determine whether resistin is capable of direct activating the expression of TNF-α in WAT, we examined its protein secretion and gene expression after exposure 24 h to rResistin. As shown in Figure 2A and 2B, the incubation of adipose tissue with rResistin resulted in the induction of TNF-α. As expected, TNF-α secretion decreased after RBP was added into the medium, indicating RBP can antagonize the role of resistin on TNF-α, but this inhibitory effect was limited to RBP with the higher concentration of 1×10–8 mol/L. Moreover, the expression change of genes was consistent with the protein level (Figure 3).
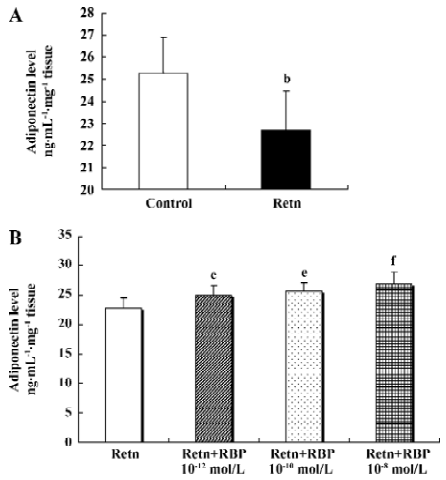
Effects of rResistin and RBP treatment on adiponectin expression in WAT Given a direct negative correlation between adiponectin and insulin resistance[14], we investigated whether resistin itself directly affects the production of adiponectin in adipose tissue. Analysis of the level of adiponectin in the culture supernatant by ELISA after treatment for 24 h with rResistin revealed decreased secretion in primary cultures of in vitro adipose tissue (Figure 4A). Consistent with the protein level, adiponectin mRNA expression was also significantly downregulated (Figure 5). In contrast, the combined treatment with RBP resulted in an increase of protein secretion (Figure 4B) and gene expression (Figure 5). Although there was no difference between the 3 concentrations of RBP, there was a concentration-dependent increase.
Discussion
Globally, the prevalence of obesity is escalating, and insulin resistance resulting from increased adipose tissue mass has been identified as a key factor that could drive parallel rises in T2DM prevalence[15]. Although it is not yet entirely clear how obesity produces insulin resistance/T2DM, it has become clear that a failure of lipid homeostasis plays an important role[16].
We suggest here that resistin, a newly found adipocyto-kine in adipose tissue, influences adipose tissue lipid metabolism. It is well known that resistin is expressed mainly in WAT in rodents. The expression of resistin mRNA in adipose tissue and protein levels in circulation were significantly elevated in diet-induced obesity mice and several obese animal models, including db/db and ob/ob mice[4]. These initial observations strongly suggest that resistin would play important roles in adipocytes and adipose tissues, and its abnormal expression would be linked to lipid metabolism disorders frequently found in obese animals. Therefore, our studies imply that overproduced and secreted resistin from obesity has direct autocrine effects on adipose tissue. Recently, many studies have suggested that overproduced FFA from adipocytes cause peripheral (muscle, liver) insulin resistance, which are linked with metabolic diseases such as T2DM and cardiovascular disease[17,18]. Thus, our experiments strongly suggest that an abnormal increase of resistin in obese subjects would promote circulation plasma FFA release, which might eventually accelerate lipolysis and contribute to the onset of insulin resistance.
In this study, we also demonstrate that administration of RBP decreases high levels of FFA, although there was no obvious correlation between FFA levels and RBP concen-tration. Recently, evidence has suggested that drugs that cause a sustained reduction in elevated plasma FFA concentration may represent an effective modality for the prevention of T2DM[19]. A similar idea has been demonstrated that the regulation of fatty acid metabolism may be a target for obesity treatment[20]. From the above points, our results indicate that RBP can antagonize the role of resistin in fatty acid metabolism and may have a potential role in improving insulin resistance by decreasing FFA release.
In spite of these findings, it has been poorly demonstrated that resistin is able to affect the functions of adipose tissues, and RBP can antagonize the roles of resistin on the development of insulin resistance. As a major tissue for whole-body energy homeostasis, many increasing studies have found that adipose tissue is also an important endocrine organ for expressing various adipocytokines, including leptin, resistin, TNF-α, adiponectin and interleukin-6[2,3]. It is well known that certain adipocytokines are important in provoking the insulin resistance found in many obese and diabetic subjects. For example, a positive relationship between obesity, insulin resistance and adipose tissue mRNA levels of TNF-α has clearly been established in rodent models[12]. An increased level of TNF-α in obese states leads to insulin resistance via a number of mechanisms, including decreased insulin-stimulated glucose transport or insulin signaling[21,22]. Unlike TNF-α, adiponectin expression levels are inversely correlated with obesity, T2DM and atherosclerosis[23,24]. Furthermore, increasing evidence has demonstrated that adiponectin is a key player in the insulin-sensitizing mechanism[14].
In this paper, we demonstrate that the expressions of TNF-α and adiponectin are changed in resistin treated adipose tissues. Treatment with resistin in WAT elevated the protein secretion and mRNA expression of TNF-α. In contrast, both protein secretion and mRNA expression of adiponectin decreased. Therefore, the observations show that resistin has a direct regulating effect on the expressions of adipocytokines in adipose tissue, but the molecular mechanisms underlying these results remain elucidated. In addition, the previous idea that resistin would induce insulin resistance in obesity was confirmed by our observation that resistin treatment changed the levels of adipocytokines in adipose tissue. Taken together, these results strongly suggest that aberrantly high expression of resistin in adipose tissues of obesity would result in insulin resistance not only by increasing FFA levels, but also by modulating adipocytokine expressions.
In accordance with the former results, our studies of RBP on the endocrine function of WAT show that RBP has an antagonizing role on resistin in adipose tissue endocrine. However, our studies failed to find a positive correlation between RBP concentration and its antagonizing role. The results of adipose tissue gene expression changes is consistent with its protein secretion, which suggests that RBP may perform its roles though the regulation of gene transcription in cells. Maybe, there were 2 mechanisms; first, as a specific bind peptide of resistin, RBP could direct combine to resistin in culture medium supernatant and inhibit the regulation effect of resistin on adipose tissue endocrine function, thereby modulates indirectly the expressions of TNF-α and adiponectin. Second, RBP compounded in this experiment is a short peptide and our previous studies have indicated that RBP is able to change the subcellular localization of resistin and inhibit resistin-induced differentiation of 3T3-L1 pre-adipocytes[10]. Thus, it is likely that RBP exerts its antagonizing on resistin through going into adipocytes directly. However, these 2 hypotheses require further investigation.
In summary, we have shown that resistin directly affects the fatty acid metabolism and adipocytokine expressions of WAT through the autocrine or paracrine pathway. Our findings that resistin treatment elevates FFA release and changes adipocytokine expression levels in adipose tissue provides strong evidence that overproduced resistin in obesity induces lipid metabolism disorders and insulin resistance. Nevertheless, this study provides the first evidence that RBP is able to antagonize these effects of resistin on adipose tissue. Further work is needed to investigate the mechanisms underlying these effects.
References
- Klyde BJ, Hirsch J. Increased cellular proliferation in adipose tissue of adult rats fed a high-fat diet. J Lipid Res 1979;20:705-15.
- Jazet IM, Pijl H, Meinders AE. Adipose tissue as an endocrine organ: impact on insulin resistance. Neth J Med 2003;61:194-212.
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548-56.
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature 2001;409:307-12.
- McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet 2002;359:46-7.
- Wolf G. Insulin resistance and obesity: resistin, a hormone secreted by adipose tissue. Nutr Rev 2004;62:389-94.
- Moon B, Kwan JJ, Duddy N, Sweeny G, Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am J Physiol Endocrinol Metab 2003;285:E106-15.
- Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by Resistin. Mol Cell Biol 2005;25:1569-75.
- Satoh H, Nguyen MT, Miles PD, Imamura T, Usui I, Olefsky JM. Adenovirus-mediated chronic “hyper-resistinemia” leads to in vivo insulin resistance in normal rats. J Clin Invest 2004;114:224-31.
- Liu F, Guo X, Gong H, Fei L, Pan X, Guo M. A resistin binding peptide selected by phage display inhibits 3T3-L1 preadipocyte differentiation. Chin Med J 2006;119:496-503.
- Canova N, Lincova D, Farghali H. Inconsistent role of nitric oxide on lipolysis in isolated rat adipocytes. Physiol Res 2005;54:387-93.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87-91.
- Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Tumor necrosis factor alpha is a negative regulator of resistin gene expression and secretion in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2001;288:27-31.
- Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003;361:226-8.
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates and projections. Diabetes Care 1998;21:1414-31.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Investing 2000;106:473-81.
- Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes 2003;111:121-4.
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002;32:S14-23.
- Bajaj M, Suraamornkul S, Kashyap S, Cusi K, Mandarino L, DeFronzo RA. Sustained reduction in plasma free fatty acid concentration improves insulin action without altering plasma adipocytokine levels in subjects with strong family history of type 2 diabetes. J Clin Endocrinol Metab 2004;89:4649-55.
- Ronnett GV, Kim EK, Landree LE, Tu Y. Fatty acid metabolism as a target for obesity treatment. Physiol Behav 2005;85:25-35.
- Sethi JK, Hotamisligil GS. The role of TNF alpha in adipocyte metabolism. Semin Cell Dev Biol 1999;10:19-29.
- Jain RG, Phelps KD, Pekala PH. Tumor necrosis factor-alpha initiated signal transduction in 3T3-L1 adipocytes. J Cell Physiol 1999;179:58-66.
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996; 271:10 697–703.
- Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, et al. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes 2001;51:2325-8.
