Transport of carbamazepine and drug interactions at blood-brain barrier1
Introduction
Epilepsy is a common condition: it affects 1% of the world’s population. Approximately 30% of epileptics are unable to adequately control their seizures with drugs[1]. Ten to twenty percent of patients with epilepsy are pharmaco-resistant, although the antiepileptic drug (AED) concentrations in these patients’ plasma are within normal therapeutic levels[2]. Many studies have shown that changes in the permeability of AED across the blood-brain barrier (BBB) may be involved in pharmacoresistance to AED[3,4]. Carbama-zepine (CBZ) is one of the most commonly used antiepileptic drugs. Transport of CBZ at the BBB may be mediated by many transporters[5,6]. However, the characteristics of CBZ transport at the BBB and its interactions with other drugs are still unclear.
In the present study, our aim was to study the characteristics of CBZ transport at the BBB, and to investigate whether some agents affect CBZ transport at the BBB by using primary cultured rat brain microvascular endothelium cells (rBMEC) as a model of the BBB.
Materials and methods
Materials CBZ was provided by Sigma Co USA, CsA was provided by the Sichuan Industrial Institute of Antibio-tics, Chengdu, China. Cosmic calf serum was purchased from Hyclone (New Zealand). Gelatin, trypsin, and collagenase type II were purchased from Sigma. Dulbecco’s modified Eagle’s medium (high glucose) (DMEM) and Ham’s F-12 nutrient mixture were purchased from Gibco BRL (Mary-land, USA). Bovine serum albumin was purchased from SABC (Fraction V; USA). Sprague-Dawley rats, (7–10 days old), were supplied by the Center of Experimental Animals, China Pharmaceutical University. All other chemicals were of analytical grade.
Isolation and culture of rBMEC[7] rBMEC were isolated from the cerebral gray matter of rat brains. The isolated cerebral gray matter was digested with trypsin (0.05%) at 37 °C for 20 min, then filtered through 149-µm nylon mesh, after which the filtrate was collected. The filtrate was filtered through 79-µm nylon mesh and the matter on the nylon mesh was collected. Then the matter was digested with type II collagenase (0.1%) at 37 °C for 20 min, centrifuged at room temperature for 5 min (200×g), and the cells were collected. The isolated rBMEC were seeded in cultured flasks coated with gelatin (2%) and cultured at 37 °C in culture medium. The culture medium (pH 7.4) consisted of high glucose DMEM and F-12 medium (1:1) containing 20% fetal bovine serum, L-glutamine (0.9 g/L), heparin (50 mg/L), streptomycin (105 U/mL), penicillin (105 U/mL) and NaHCO3 (0.5 g/L). On the 4th day after seeding, the culture medium was replac-ed, after which the culture medium was replaced every 2 d. On d 12, the rBMEC were transferred to 24-well plates coated with gelatin (2%) and cultured at 37 °C for 12−14 d. Transport experiments were performed when the cells reached confluence. Cultured rBMEC were identified by an immuno-staining method using factor-VIII related antigen.
Transport experiment When cells reached confluence after 12−14 d, uptake experiments were performed. Briefly, the cultured cell monolayer was washed 3 times with 1 mL of pH 7.4 Hanks’ solution (isotonic buffer) containing NaCl (0.137 mol/L), KCl (5.37 mmol/L), CaCl2 (1.26 mmol/L), MgSO4·7H2O (0.81 mmol/L), Na2HPO4·H2O (0.37 mmol/L), KH2PO4 (0.44 mmol/L), NaHCO3 (4.17 mmol/L), and glucose (2.92 mmol/L) at 37 ºC. The cultured cells were pre-incubated at 37 °C for 30 min in Hanks’ solution. After the pre-incuba-tion, the solution was removed by suction, and Hanks’ solution (0.3 mL) containing both CBZ (10 mg/L) and the agents for testing was added to the incubation wells. To terminate the transport reaction, cells were washed 3 times with 0.5 mL ice-cold Hanks’ solution at the designated time. Then, 0.2 mL ultrapure water was added to each incubated well, and the material in the wells was frozen and melted repeatedly 3 times to break down the cells[7].
Efflux experiment For the efflux study, rBMEC were incubated with 10 mg/L CBZ at 37 °C for 30 min, then the cells were washed 3 times with 1 mL ice-cold Hanks’ solution. Then Hanks’ solution with or without 100 μmol/L olanzapine (OLZ), 50 μmol/L novobiocin (NO), or 50 μmol/L CsA was added to initiate the efflux of CBZ at 37 °C. Termination of the efflux was performed by using the same procedure as used in the uptake study described earlier. Efflux was estimated from the amount of CBZ remaining in the cells[8].
Analytical method The concentrations of CBZ in the cells were determined by using LC-MS (liquid chromatography mass spectrometry)[9]. The sensitivity of the assay was 0.39 µg/L. Reproducibility was better than 95% in the tested ranges and good linearity was obtained from 0.39 µg/L to 50 µg/L. Protein content in the cultured cells was measured by using the Bradford method,[10] using bovine serum albumin as the standard. Net uptake, expressed as the ratio of CBZ concentration to protein concentration (ng/µg protein), was obtained by dividing the apparent amount of CBZ taken up by the amount of protein (µg). All the results are provided as mean±SD. Student’s t-test was used for statistical analysis and statistical significance was set at P<0.05 or P<0.01.
Results
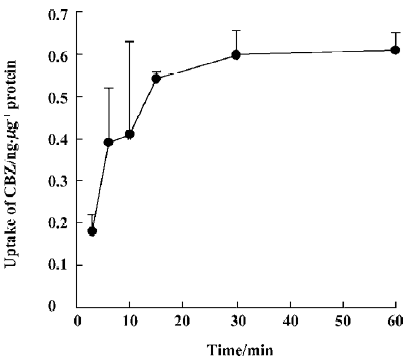
CBZ uptake over time The accumulation of CBZ is time-dependent and a plateau of accumulation was observed between 15 min and 60 min. Accordingly, uptake at 30 min was used for experiments evaluating the effects of other drugs on the transport characteristics of CBZ (Figure 1).
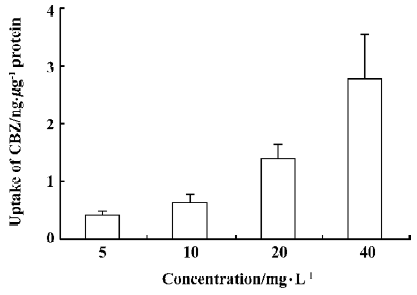
Uptake of CBZ at different concentrations After cells were incubated with a medium containing 5, 10, 20, or 40 mg/L CBZ at 37 °C for 30 min, the amounts of CBZ taken up by cells were measured (Figure 2). We found that the uptake of CBZ by rBMEC was concentration-dependent, but that the ratio of the concentration in cells to that in the medium was similar for all concentrations, indicating that the uptake of CBZ is linear between concentrations of 5 mg/L and 40 mg/L.
Effects of other drugs on the steady-state uptake of CBZ CBZ uptake (UT) by cells incubated with a medium containing 10 mg/L CBZ and various other agents was measured at 37 °C for 30 min. The percentage change (Δ%) compared with the control (UC) was calculated according to the following equation:
Δ% = (UT/UC–1)×100%, (1)
and the calculated results are shown in Figure 3.
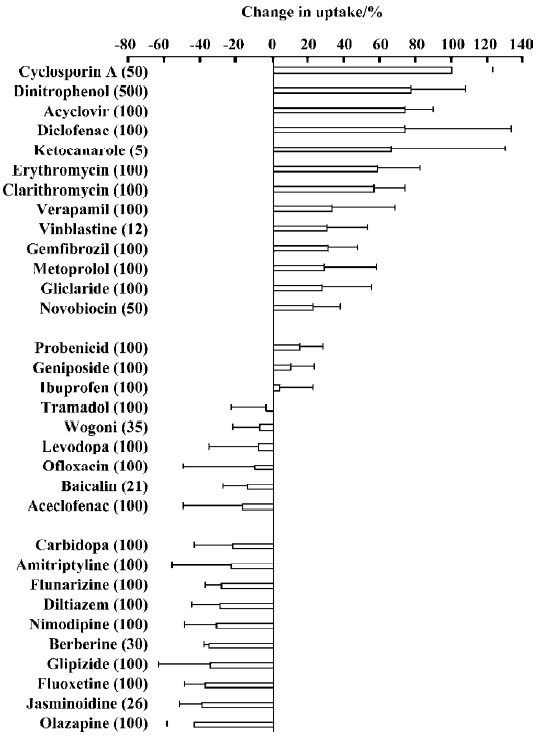
The agents tested can be divided into 3 groups based on the percentage change in the steady-state uptake of CBZ. Group 1 agents, cyclosporin A, dinitrophenol, erythromycin, clarithromycin, acyclovir and diclofenac, increased the uptake of CBZ. The percentage uptake increased by more than 20% for all group 1 agents. Dinitrophenol, a metabolic inhibitor, increased CBZ uptake significantly, which indicated that some energy-dependent efflux transporters may be involved in the transport of CBZ. Group 2 agents, OLZ, jasminoidin and fluoxetine, decreased the uptake of CBZ by more than 20%. Group 3 agents, levodopa and ibuprofen, had little effect on the steady-state uptake of CBZ.
In order to investigate whether the changes in CBZ uptake by cells in presence of the tested agents resulted from cell damage caused by the agents used, cells were incubated at 37 °C for 30 min with 10 mg/L CBZ, and 50 μmol/L CsA, 50 μmol/L novobiocin, 100 μmol/L aciclovir, 100 μmol/L diclofenac or 100 μmol/L OLZ. The cell activity was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. No differences were found relative to the control. Thus, we believed that the agents used did not damage cells at the concentrations used, and that changes in the uptake of CBZ did not result from cell damage.
Two typical agents, CsA (which increased the uptake of CBZ) and OLZ (which decreased the uptake of CBZ) were selected for further study.
Effect of CsA on the uptake of CBZ by rBMEC The uptake of CBZ by rBMEC in the presence of 50 μmol/L CsA, at both 37 °C and 4 °C, is shown in Figure 4. CsA significantly (P<0.01) increased the uptake of CBZ at 37 °C, but not at 4 ºC.
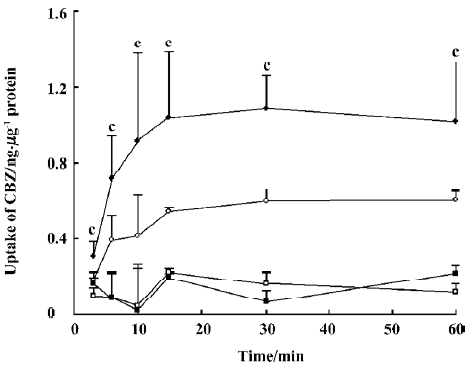
Effect of OLZ on the uptake of CBZ The effects of different concentrations of OLZ on the uptake of CBZ by cells were measured after incubation for 30 min at both 37 °C and 4 °C (Figure 5).
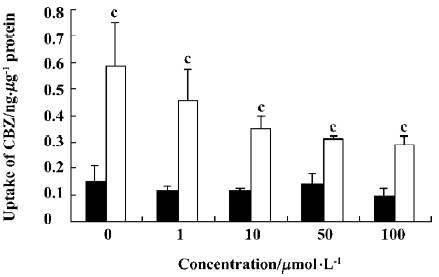
OLZ decreased the uptake of CBZ in a concentration-dependent manner from 1 to 100 µmol/L at 37 °C. However, OLZ did not influence the uptake of CBZ at 4 °C.
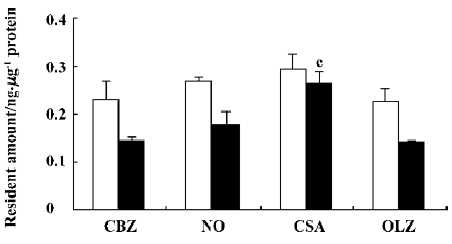
Effect of CsA and OLZ on the efflux of CBZ from rBMEC The results described earlier showed that CsA increased the uptake of CBZ, whereas OLZ decreased the uptake of CBZ. In order to investigate whether CsA and OLZ influenced the uptake of CBZ via the efflux of CBZ from rBMEC, the efflux of CBZ from rBMEC after 30 min and 60 min in the presence of the tested agents was studied, and results are shown in Figure 6. CsA significantly (P<0.01) decreased the efflux of CBZ from rBMEC. The BCRP (breast cancer resistance protein, BCRP) inhibitor, novobiocin, also slightly decreased the efflux of CBZ. OLZ did not influence the efflux of CBZ.
Discussion
The present study showed that the uptake of CBZ by rBMEC was time-dependent, concentration-dependent, temperature-dependent, and energy-dependent. Many trans-porters, including ABC efflux transport systems, may be involved in the uptake of CBZ by rBMEC. Furthermore, we found that drugs influenced the uptake of CBZ in different ways, and that the drugs we tested could be divided into 3 groups.
Most of the agents in group 1 may be involved in one or more ABC transporters. For example, CsA, a common P-gp inhibitor, also inhibits BCRP transporters[11]. Erythromycin, clarithromycin, and verapamil are inhibitors of P-gp[7], and erythromycin also affects OAT(organic anion transporter)transporters[12]. Aciclovir may affect MRP(multidrug resistance-associated protein, MRP) transporters[13]. Gemfibrozil can be transported by P-gp and MRP transporters[14], and novobiocin is an inhibitor of BCRP transporters[15]. CsA increased the uptake of CBZ by rBMEC in a concentration-dependent and temperature-dependent manner, which may occur via inhibiting the efflux of CBZ from rBMEC.
Diltiazem, nimodipine and berberine in group 3 did not increase the uptake of CBZ by rBMEC, although they are all substrates of P-gp. In contrast, these 3 agents tended to decrease the uptake of CBZ. Andrew et al reported that CBZ was not a substrate of P-gp in a Coca-2 model, or in mdr1a/1b(-/-) mice[5]. A similar result was found by Maines et al using bovine retinal endothelial cells[16]. These results suggest that P-gp is not likely to modulate CBZ transport in rBMEC, and that other ABC transporters are involved in the efflux of CBZ from rBMEC.
OLZ, jasminoidin and fluoxetine significantly inhibited the uptake of CBZ by rBMEC. OLZ inhibited the uptake of CBZ by rBMEC in a concentration-dependent and temperature-dependent manner. Further study showed that OLZ did not affect the efflux of CBZ from rBMEC. These results indicate that some transporters may be involved in the influx of CBZ in rBMEC, and that OLZ may decrease uptake in CBZ via inhibiting these transporters.
Together, these observations indicate that the transport of CBZ at the BBB is mediated by many transporters. Some specific ABC efflux transporters may be involved in the transport of CBZ. Various drugs influence the transport of CBZ at the BBB in different ways.
References
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314-9.
- Dombrowski SM, Desai SY, Marroni M. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 2001;42:1501-6.
- Loscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther 2002;301:7-14.
- Rogawski MA. Does P-glycoprotein play a role in pharmaco-resistance to antiepileptic drugs? Epilepsy Behav 2002;3:493-5.
- Andrew O, Munir P, Justice N. Carbamazepine is not a substrate for P-glycoprotein. Clin Pharmacol 2001;51:345-9.
- Sisodiya SM, Lin WR, Harding BN, Squier MV, Thom M. Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain 2002;125:22-31.
- Zhang L, Liu XD. P-glycoprotein restricted transport of nimodi-pine across blood-brain barrier. Acta Pharmacol Sin 2003;24:903-6.
- Tsuji A, Terasaki T, Takabatake Y, Tenda Y, Tamai I, Yamashima T, et al. P-Glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci 1992;151:1427-37.
- Sun JJ, Liu XD, Xie L. Determination of carbamazepine in the rat brain capillary endothelial cells by LC-MS method. J China Pharm Univ 2005;36:241-4.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.
- Tanjia E, Sabine H, Hans-Joachim G. Characterisation of the brain multidrug resistance protein expressed at the blood-brain barrier. Brain Res 2003;971:221-31.
- Kobayashi Y. Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. Mol Pharmacol 2002;62:7-14.
- Wada S. Rat multispecific organic anion transporter 1 (rOAT1) transports zidovudine, acyclovir, and other antiviral nucleoside analogs. J Pharmacol Exp Ther 2000;294:844-9.
- Kivisto KT. Characterisation of cerivastatin as a P-glycoprotein substrate: studies in P-glycoprotein-expressing cell monolayers and mdr1a/b knock-out mice. Naunyn Schmiedebergs Arch Pharmacol 2004;370:124-30.
- Shiozawa K, Oka M, Yoshikawa M. Reversal of breast cancer resistance protein-mediated drug resistance by novobiocin, a coumermycin antibiotic. Int J Cancer 2004;108:146-51.
- Maines LW, Antonetti DA, Wolpert EB, Smits CD. Evaluation of the role of P-glycoprotein in the uptake of paroxetine, cloza-pine, phenytoin and carbamazepine by bovine retinal endothelial cells. Neuropharmacology 2005;49:610-7.
