Association of CYP2D6 and CYP1A2 gene polymorphism with tardive dyskinesia in Chinese schizophrenic patients
Introduction
Tardive dyskinesia (TD) is a late-occurring effect of the long-term use of antipsychotic medication for the treatment of schizophrenia. It is characterized by excessive and involuntary movements, which can be persistent, and cause difficulties in breathing, speech and mood. Several hypotheses have been proposed regarding the pathophysiology of TD, including dopamine receptor hypersensitivity[1], GABA insufficiency[2], and structural abnormalities[3]. However, the pathophysiology of TD is still not completely understood.
The cytochrome P450 enzyme debrisoquine/spartein-hydroxylase (CYP2D6 and CYP1A2) plays an important role in the metabolism of neuroleptic drugs[4]. CYP2D6 is considered a high-affinity, low-capacity metabolic clearance pathway for typical antipsychotic drugs, whereas CYP1A2 is considered to be a low-affinity, high-capacity metabolic clearance pathway[5]. These enzymes have been shown to have a gene-dose effect on the pharmacokinetics and clinical effects of neuroleptic drugs[6,7]. Genetic differences in CYP2D6 and CYP1A2 may mean that some patients have only less active enzymatic forms, resulting in higher plasma levels of the medication, and this may also allow prediction of good response and propensity to side-effects. Polymorphisms have been noted in the genes of these 2 enzymes[8,9]. Although more than 50 variant alleles have been reported to date, most of them occur at rare frequencies and may not be of practical significance. The CYP2D6 C100T polymorphism and the CYP1A2 C163A polymorphism are some of the most frequent gene mutations in Asian populations[10]. In addition, there are numerous reports of an association between the C100T and C163A polymorphisms and TD[4,11,12]. However, the results of association analyses are still contradictory[13,14], and little is known about the potential existence of an association in Chinese people.
In the present study, we investigated the association between TD and the C100T polymorphism of the CYP2D6 gene and the C163A polymorphism of the CYP1A2 gene.
Materials and methods
Subjects Patients with schizophrenia were recruited from Guangzhou Psychiatric Hospital. All subjects were diagnosed with schizophrenia according to DSM-IV (Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition) criteria by 2 psychiatrists who made independent assessments based on case records and interviews with patients. Evidence of TD was evaluated by using the Abnormal Involuntary Movement Scale (AIMS)[15]. In the AIMS, dyskinesia is assessed in different regions of the body, including the facial and oral regions, the extremities, and the trunk. Severity ratings were allocated using a 5-point ordinal scale from 0 to 4 (corresponding to none, minimal, mild, moderate and severe, respectively). Patients were considered to have TD if there were at least moderate or severe abnormal involuntary movements in 1 or more of the 7 body areas examined, or at least mild movements in 2 or more areas. Initially, the patients were divided into TD and non-TD groups. The TD and non-TD groups were further evaluated by AIMS on 3 occasions over 6 months. Finally, a total of 91 patients with persistent TD were defined as the TD group, and 91 patients who were consistently without dyskinesis were defined as the non-TD group. Each subject received a general medical examination, and we also collected information on patient age, duration of illness, and cumulative exposure to neuroleptic drugs in years. It was established that all patients had been treated with typical antipsychotics from at least one chemical class for a minimum of 1 year. The subjects were genetically unrelated, and informed consent was obtained from them or their guardians.
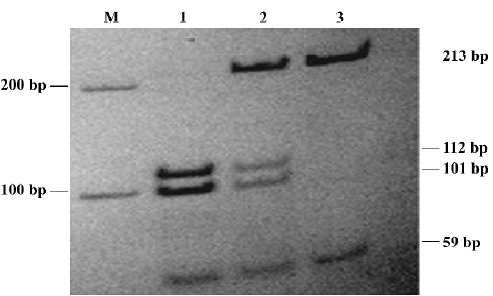
Genotyping Genomic DNA was extracted from peripheral blood lymphocytes by using the standard phenol-chloroform method. A 272 bp fragment containing the C100T polymorphism in exon 1 of the CYP2D6 gene was amplified by polymerase chain reaction (PCR) using the forward primer 5'-CCATTTGGTAGTGAGGCAGGTAT-3' and the downstream primer 5'-CCATCCATGTTTGCTTCTGGT-3'. PCR was amplified as described by Garcia-Barcelo[16] . The resulting PCR products were incubated with 10 U HphI in the recommended buffer at 37 °C overnight. Then the digested products were electrophoresed on 12% polyacrylamide non-denaturing gel, and stained with silver. The C allele was represented by 213 bp and 59 bp fragments, and the T allele was represented by 4 fragments: 213, 112, 101, and 59 bp.
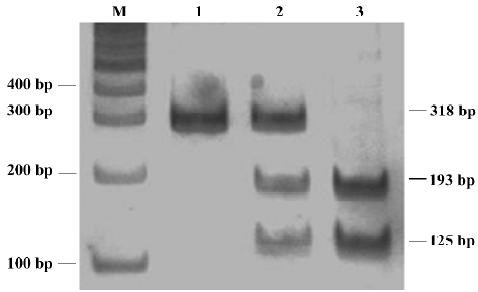
The C163A polymorphism in the CYP1A2 gene was detected based on the method of Matsumoto et al[17]. A 318 bp product was obtained by using the forward primer 5'-CTACTCCAGCCCCAGAAGTG'-3 and the reverse primer 5'-GAAGGGAACAGACTGGGACA-3'. PCR products were digested with 10 U Bsp120I at 37 °C overnight. Then the digested products were electrophoresed on 12% polyacrylamide non-denaturing gel, and stained with silver. The A allele yielded an uncut single 318 bp band, whereas the C allele was represented by a 193 bp band and a 125 bp band.
Statistical analysis Statistical analysis was performed with the SPSS 8.0 software package. Allele frequencies were determined by counting alleles and calculating sample proportions. Hardy-Weinberg equilibrium was confirmed by using the χ2 test. Differences of genotypic and allelic distribution between groups were analyzed by using the χ2 or Fisher’s exact tests (when appropriate). Differences between groups with respect to age, duration of illness and cumulative exposure to neuroleptic drugs in years were assessed using the Mann-Whitney U-test. Differences between groups with respect to sex and smoking habits were assessed by using the χ2 test. One-way ANOVA was used to compare the mean AIMS scores for each of the genotypic classes. Logistic regression was used to determine whether the CYP2D6 T allele or the CYP1A2 C allele are additional risk factors for TD. Covariates included age, sex, duration of illness, and CYP2D6 and CYP1A2 genotype.
Results
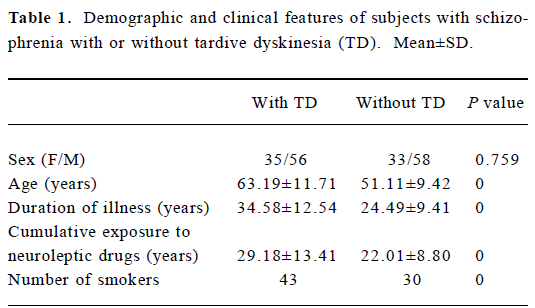
Demographic and clinical features of subjects The demographic and clinical data for the patients are summarized in Table 1. There was no significant difference in the female/male ratios between the TD and non-TD groups (P=0.759); however, age, duration of illness, cumulative exposure to neuroleptic drugs (in years) and the number of smokers were significant variables which had to be adjusted for in subsequent logistic analyses.
Full table
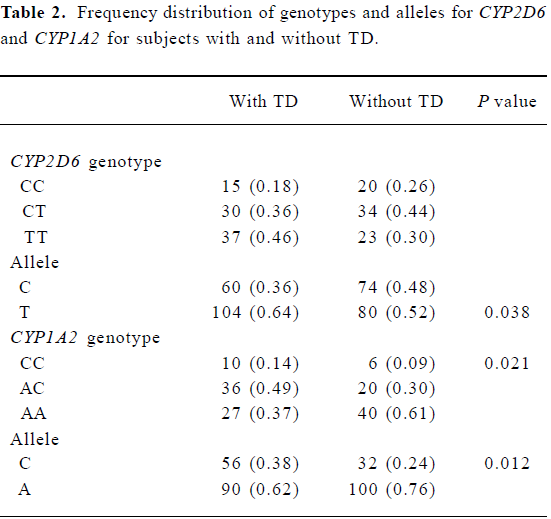
Allele and genotype frequencies The CYP2D6 C100T genotype and the CYP1A2 C163A genotype were determined by using PCR-RFLP (Figures 1,2). The distribution of alleles and genotype frequencies for C100T and C163A in schizophrenic patients is shown in Table 2. No allele frequencies deviated from Hardy-Weinberg equilibrium.
Full table
Association between genotype and TD The frequency of the CYP2D6 T allele was 64% in patients with TD and 52% in those without TD. A significantly higher frequency of the CYP2D6 T allele was found in the TD group relative to the non-TD group (χ2=4.28, P<0.05; OR= 1.60, 95%CI: 1.024–2.510). The frequency of the CYP1A2 C allele was 38% in the TD group and 24% in the non-TD group (Table 3). A significantly higher frequency of the CC genotype and the CYP1A2 C allele was seen in the TD group relative to the non-TD group (χ2=7.76, P<0.05 for CC genotype; χ2=6.38, P<0.05 for C allele; OR=1.94, 95% CI: 1.16–3.27). Thus, the CYP2D6 gene C100T polymorphism and the CYP1A2 gene C163A polymorphism were associated with TD.
AIMS scores and genotype Results from one-way ANOVA analysis of AIMS scores against genotype class suggested that there was no differences in the mean AIMS scores with respect to different genotypes either in all TD patients or in smokers only.
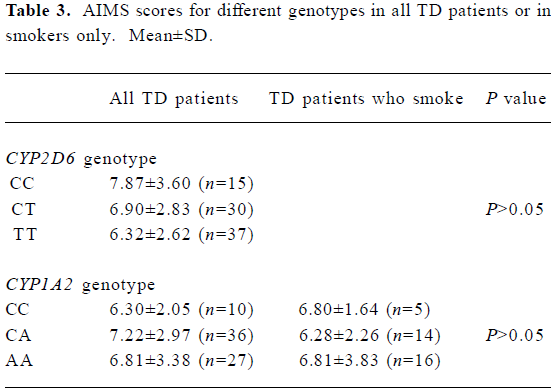
Full table
Estimation of risk for TD Because mean age, duration of illness and cumulative exposure to neuroleptic drugs (in years) were significant variables in the two study groups, we used logistic regression analysis to determine whether any of sex, age, duration of illness, cumulative exposure to neuroleptic drugs (in years), and CYP2D6 and CYP1A2 genotype are risk factors for TD. Mean age and duration of illness were included in the logistic regression equations, but not sex, cumulative exposure to neuroleptic drugs, or CYP2D6 or CYP1A2 genotype.
Discussion
We found that the frequency of the CYP2D6 T allele in TD patients in the present study (64%) was higher than the figure of 52% determined in a Japanese study[18]. For the CYP1A2 C163A polymorphism, the frequency of the C allele in our study was 62%, which is similar to the figure of 66% found in an American population, but higher than the figures of 52% and 49.7% found in an African-American[5] and a Japanese[16] population, respectively. Thus, there are marked interethnic differences in the frequencies of these polymorphisms.
The CYP2D6 gene is located on chromosome 22, and is known to be polymorphic. The C100T polymorphism in this gene is a C100→T mutation that causes a Pro34→Ser amino acid substation, leading to the formation of an unstable enzyme with lower metabolic activity. A possible correlation between TD and the CYP2D6 C100T polymorphism was previously examined by Ohmori et al[17]. These authors studied 100 Japanese subjects with schizophrenia who suffered from TD due to long-term treatment with neuroleptic drugs. There was a significant difference in the allelic distribution of CYP2D6 C100T between subjects with and without TD. In the present study, the CYP2D6 C100T genotype was found to have a significant association with the total AIMS score, and a modest association with TD occurrence. Similar results were reported by Liou et al[11], who studied movement disorders in Chinese patients with schizophrenia from Taiwan, China. They also found a modest association between TD and C100T genotype, but this positive finding was only observed in male patients. Moreover, their findings also support the correlation between AIMS score and C100T polymorphism within the TD group. In the present study, no significant differences in the genotype frequency of the CYP2D6 C100T polymorphism were observed between patients with and without TD. However, patients with TD had a significantly greater frequency of the 100T allele compared with those without TD. Our results indicate that CYP2D6 C100T polymorphism may be associated with TD.
The CYP1A2 gene is located on chromosome 15. A recent study in a sample of 236 healthy Caucasian volunteers identified a single nucleotide polymorphism (C→A) in intron 1 of the CYP1A2 gene at position 734, downstream from the transcription initiation site. In that sample, subjects homozygous for the C/C genotype had, on average, 40% lower CYP1A2 activity as measured by a plasma caffeine metabolic ratio, in comparison with those homozygous for the A variant[19]. In the present study, patients with TD had a significantly greater frequency of CC genotype and C allele compared with those without TD, which was consistent with the findings of Basile et al[5], but the median AIMS scores did not differ between individuals with the A/A, A/C, or C/C genotypes. This, in our opinion, could be explained by small sample size or differential pathology of TD in different racial groups.
The results of logistic regression analysis demonstrated that mean age and duration of illness were risk factors for TD, but that, sex, cumulative exposure to neuroleptic drugs (in years), and CYP2D6 and CYP1A2 genotype were not, which suggests that genetic factors have a weaker association with susceptibility to TD compared with mean age and duration of illness.
It is necessary to address the fact that this study had some limitations. First, the number of cases were limited, thus there was low statistical power. Second, because mean age, duration of illness and cumulative exposure to neuroleptic drugs (in years) were significant variables in the groups with and without TD, the samples of the case-control study were not fully pair-matched, which may produce false positive results. Third, although smoking status was confirmed in 165 patients in the sample, detailed information on smoking habits was unavailable. The extent of exposure to tobacco smoke positively correlates with CYP1A2 activity[20], and smoking may also alter the risk for TD via a pharmacodynamic effect of nicotine[21]. Therefore, interindividual differences in smoking habits in our sample might have biased our observations. Fourth, the C→A polymorphism may exert a direct effect on the development of TD or, alternatively, the polymorphism may be in linkage disequilibrium with a nearby regulatory sequence such as a (G→A) polymorphism in the 5'-flanking region of the CYP1A2 gene at position 2964 from the transcription initiation site[22]. Further studies are warranted to test the association between this CYP1A2 (G→A) genetic polymorphism and TD risk in Asian populations.
In conclusion, the present study indicates that the CYP2D6 T allele and the CYP1A2 C allele may be risk factors for TD in Chinese people. Additional studies with a larger sample size will be required to confirm our results in populations comprising other racial groups, and to clarify the exact role of the CYP2D6 and CYP1A2 genes with regard to risk for TD.
References
- Christensen AV, Nielsen IM. Dopaminergic supersensitivity: influence of dopamine agonists, cholinergics, anticholinergics, and drugs used for the treatment of tardive dyskinesia. Psychopharmacology (Berl) 1979;62:111-6.
- Gasey DE. Tardive dyskinesia: pathophysiology and animal models. J Clin Psychiatry 2000;61 Suppl 4:5-9.
- Dalgalarrondo P, Gattaz WF. Basal ganglia abnormalities in tardive dyskinesia. Possible relationship with duration of neuroleptic treatment. Eur Arch Psychiatry Clin Neurosci 1994;244:272-7.
- Brosen K. Drug-metabolizing enzymes and therapeutic drug monitoring in psychiatry. Ther Drug Monit 1996;18:393-6.
- Basile VS, Ozdemir V, Masellis M. A functional polymorphism of the cytochrome P450 1A2 (CYP1A2) gene: association with tardive dyskinesia in schizophrenia. Mol Psychiatry 2000;5:410-7.
- Meyer UA. Pharmacogenetics: the slow, the rapid, and the ultrarapid. Proc Natl Acad Sci USA 1994;91:1983-4.
- Ball SE, Scatina J, Kao J. Population distribution and effects on drug metabolism of a genetic variant in the 5' promoter region of CYP3A4. Clin Pharmacol Ther 1999;66:288-94.
- Garcia-Barcelo M, Chow LY, Chiu HF. Genetic analysis of the CYP2D6 locus in a Hong Kong Chinese population. Clin Chem 2000;46:18-23.
- Hamdy SI, Hiratsuka M, Narahara K. Genotyping of four genetic polymorphisms in the CYP1A2 gene in the Egyptian population. Br J Clin Pharmacol 2003;55:321-4.
- Wang SL, Huang JD, Lai MD, Liu BH, Lai ML. Molecular basis of genetic variation in debrisoquine hydroxylation in Chinese subjects: polymorphism in RFLP and DNA sequence of CYP2D6. Clin Pharmacol Ther 1993;53:410-5.
- Liou YJ, Wang YC, Bai YM. Cytochrome P-450 2D6*10 C100T polymorphism is associated with antipsychotic-induced persistent tardive dyskinesia in Chinese schizophrenic patients. Neuro-psychobiology 2004;49:167-73.
- Lohmann PL, Bagli M, Krauss H. CYP2D6 polymorphism and tardive dyskinesia in schizophrenic patients. Pharmacopsychiatry 2003;36:73-8.
- Schulze TG, Schumacher J, Muller DJ, Krauss H, Alfter D, Maroldt A. Lack of association between a functional polymorphism of the cytochrome P450 1A2 (CYP1A2) gene and tardive dyskinesia in schizophrenia. Am J Med Genet 2001;105:498-501.
- Tiwari AK, Deshpande SN, Rao AR, Bhatia T, Lerer B, Nimgaonkar VL. Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: III. Lack of association of CYP3A4 and CYP2D6 gene polymorphisms. Schizophr Res 2005;75:21-6.
- Schooler NR, Kane JM. Research diagnosis of tardive dyskinesia. Arch Gen Psychiatry 1982;39:486-7.
- Garcia-Barcelo M, Chow LY, Chiu HF, Wing YK, Lee DT, Lam KL, et al. Genetic analysis of the CYP2D6 locus in a HongKong Chinese population. Clin Chem 2000;46:18-23.
- Matsumoto C, Ohmori O, Shinki T. Genetic association analysis of functional polymorphisms in the cytochrome P450 1A2 (CYP1A2) gene with tardive dyskinesia in Japanese patients with schizophrenia. Psychiatr Genet 2004;14:209-13.
- Ohmori O, Suzuki T, Kojima H. Tardive dyskinesia and debriso-quine 4-hydroxylase (CYP2D6) genotype in Japanese schizo-phrenics. Schizophr Res 1998;32:102-13.
- Sachse C, Brockmoller J, Bauer S. Functional significance of C¡úA polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 1999;47:445-9.
- Kalow W, Tang BK. Caffeine as ametabolic probe: exploration of the enzyme-induced effect of cigarette smoking. Clin Pharmacol Ther 1991;49:44-8.
- Kirch DG, Alho AM, Wyatt RJ. Hypothesis: a nicotine-dopamine interaction linking smoking with Parkinson’s disease and tardive dyskinesia. Cell Mol Neurobiol 1988;8:285-91.
- Nakajima M, Yokoi T, Mizutani M. Genetic polymorphism in the 5-flanking region of the CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem 1999;125:803-8.
