Effect of Oenanthe javanica flavone on human and duck hepatitis B virus infection1
Introduction
Oenanthe javanica (Oj), umbelliferate, has been widely used in traditional Chinese medicine for treatment of jaundice, hypertension, and polydipsia diseases for many years[1]. Previous studies have shown that it has liver-protective[2], hypotensive[3], anti-arrhythmic[4], anti-anaphylactic[5], and antidiabetic[6] effects. Recent studies also show that Oj was helpful in treatment of hepatitis B virus (HBV) infection in clinical trials[7], and had an inhibitory effect on duck hepatitis B virus (DHBV)-induced hepatitis in Nestling ducks in vivo[8] and in vitro[9]. However, which is the active part and what are the active components of Oj in inhibiting HBV remain unclear. Using modern techniques, it has been revealed that OjF is one of the main active parts against HBV and it comprises approximately 2.2% of the whole plant content. The present study aimed to investigate the anti-hepatitis activity of OjF in 2.2.15 cells in vitro and duck HBV infection in a duck model in vivo.
Materials and methods
Preparation of OjF Oj was collected from the Yanbian Autonomus Region in autumn and identification was performed by Prof Hui-zhong XIAO, Department of Phyto-chemistry, Yanbian Medical University. Dried whole plant was pulverized and extracted with 80% ethanol, and subsequently partitioned in ethyl acetate. The ethyl acetate soluble materials were fractionated by ethanol and distilled water gradient of column chromatography in polyamide. OjF was eluted and the content was determined according to aluminium nitrate reagent method. The content of hyperoside, the major ingredient of OjF, was determined by reverse phase-high performance liquid chromatography (RP-HPLC)[10,11]. The contents of OjF and hyperoside were 51.67% and 7% in whole extracts, respectively. The concentration used in the experiment was based on the dry weight of the extract.
Experimental animals Beijing ducklings within 1 d of hatching were obtained from an animal breeding farm, Chinese Academy of Medical Sciences [SCXK- (Beijing) 2002-001].
Reagents Minimum essential medium (MEM) was obtained from Gibco BRL (Gaithersburg, MD, USA). Fetal bovine serum (FBS) and G-418 were purchased from HyClone (Logan, Utah, USA). L-glutamine was obtained from Sigma (St Louis, MO, USA). HBsAg and HBeAg enzyme immunoassay (EIA) kits were purchased from China Isotope Co (Beijing, China).
Cell culture and treatment HepG2.2.15 cells (clonal cells derived from human hepatoma cell line G2) were from the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences. The 2.2.15 cells were cultured in complete MEM (containing 10% FBS, 100 kU/L benzylpenicillin, streptomycin, G-418, L-glutamine 0.03%, pH 7.0) in 75-cm2 tissue culture flasks at 37 ºC in a humidified 5% CO2.
Cytotoxic studies The 2.2.15 cells were first seeded into 96-well plates (Corning Inc, Corning, NY, USA) at a density of 1.0×105 cells per mL and cultured in 200 µL complete MEM containing 10% FBS. After 24 h of incubation, cells were washed three times with phosphate-buffered |saline (pH 7.0) and treated with different concentrations (0.125, 0.25, 0.50, 1.00, 2.00, and 4.00 g/L) of OjF in serum-free medium for 12 d. The medium was replaced every 4 d in MEM supplemented with various concentrations of OjF. Untreated cells were used as control. The cell pathological changes (CPE) were observed by microscope. The degree of CPE was graded as: all positive cells (–), the number of negative cells <25% (+), 25%–49% (++), 50%–75% (+++), and >75% (++++).
Determination of HBsAg and HBeAg The 2.2.15 cells were incubated in 24-well plates at a density of 1.0×105 cells per mL in 1 L MEM medium containing 10% FBS. After 24 h, the 2.2.15 cells were treated with different concentrations of OjF (0.125, 0.25, 0.50, and 1.00 g/L) in serum-free medium. Cells grew in the presence of drugs for 9 d with changes of medium every 3 d. After 6 and 9 d, supernatant was collected and performed at -20 °C. The HBsAg and HBeAg in culture medium were simultaneously measured by EIA kits on d 6 and d 9.
Experimented animals infection and drug treatment Beijing ducklings within 1 d of hatching were inoculated intravenously with DHBV-DNA-positive serum from Shanghai ducks (0.2 mL/animal). Seven days after infection, ducklings were divided into five groups: the control group (normal saline); the positive drug group (ACV, 0.1 g·kg-1·d-1); and the OjF 0.25, 0.50, and 1.00 g·kg-1·d-1 groups. Drugs were administered orally, bid for 10 d. Serum samples were obtained before treatment (d 0), d 5, and d 10 during treatment, and d 3 (d 13) after the cessation of treatment. The serums were stored at -70 °C for future analysis.
Viremia analysis Viremia was assessed throughout the treatment and follow-up period by a semi-quantitative detection of DHBV-DNA in duck serum using a dot-blot hybridization. Fifty microliters of serum was spotted directly onto nitrocellulose filters. After denaturation and neutralization, the filters were hybridized with a full-length DHBV genomic DNA probe labeled with 32P. The filters were autoradio-graphed and the spots were counted in a scintillation counter.
Histopathological examination of hepatocytes On d 13, each duckling was laparotomized to obtain the liver immediately after collecting blood from the leg vein. Fragments of the ducklings liver were fixed in 10% formalin solution, dehydrated with ethanol solution from 50% to 100%, embedded in paraffin and cut into 5 µm sections, and stained using haematoxylin-eosin dye for photomicroscopic observations.
Statistics The data were expressed as mean±SD, and analyzed by one-way repeated-measure ANOVA and t-test for comparisons between groups. P<0.05 was considered statistically significant.
Results
The cytotoxicity of OjF in 2.2.15 cells OjF-induced cytotoxicity was observed by microscope. After 12 d of incubation with 1.00 g/L OjF, no significant difference was found from that of the control. However, when OjF concentration increased, cell injury caused by OjF was observed (Table 1). The 50% toxic concentrations (TC50) was 2.28±0.13 g/L and the maximum nontoxic concentrations (TC0) was 1.00 g/L.
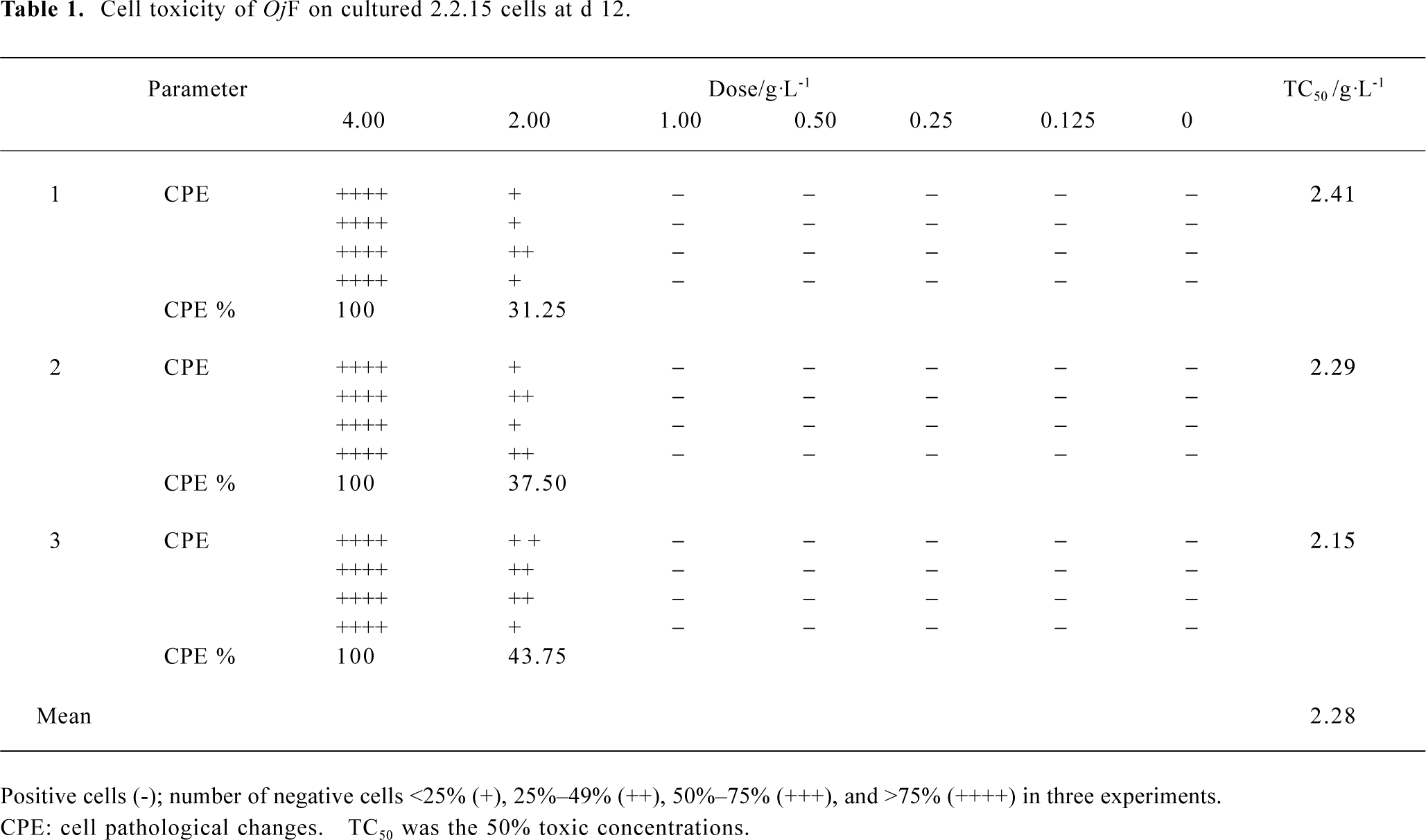
Full table
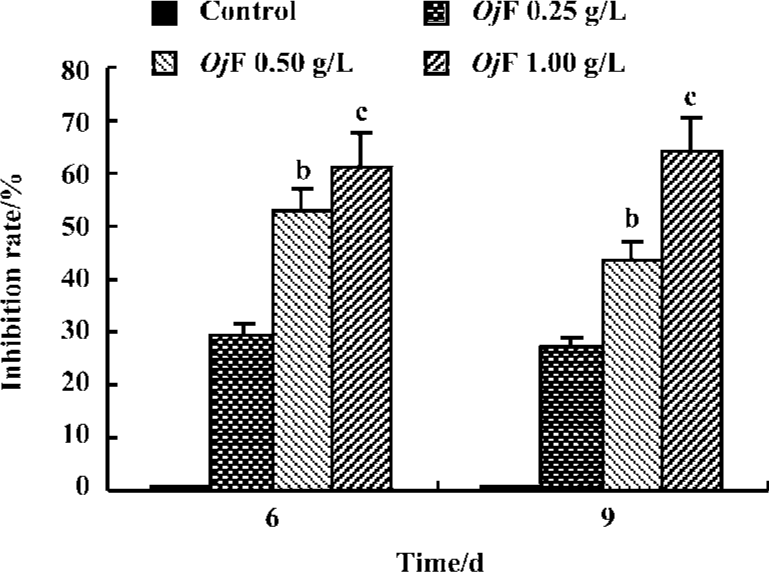
Inhibition of HBsAg and HBeAg production in 2.2.15 cells After 9 d of incubation, HBsAg and HBeAg production in the culture medium were determined (Table 2). The results showed that OjF suppressed HBsAg and HBeAg production in the 2.2.15 cells with median effective concentration (IC50) of about 0.56 and 0.41 g/L respectively on d 6, 0.64 and 0.30 g/L respectively on d 9 (Table 3). OjF-induced suppression of HBsAg and HBeAg production in 2.2.15 cells was also reflected by the inhibition rate percentage (Figure 1, 2). The inhibitory effects appeared when cells were treated with 0.25 g/L OjF. A substantial increase of inhibitory effects was observed from 0.50 g/L OjF. When treated with 1.00 g/L OjF, the inhibition rate percentage on HBsAg and HBeAg in 2.2.15 cells were both more than 50%. The inhibition rate percentage on HBsAg was dose-dependent, and the inhibition rate percentage on HBeAg was both time- and dose-dependent.
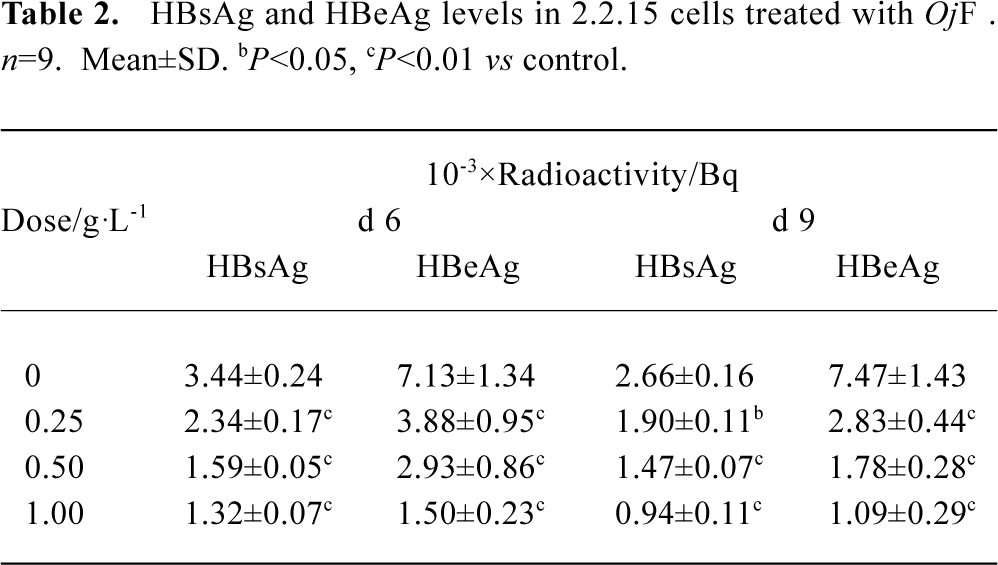
Full table
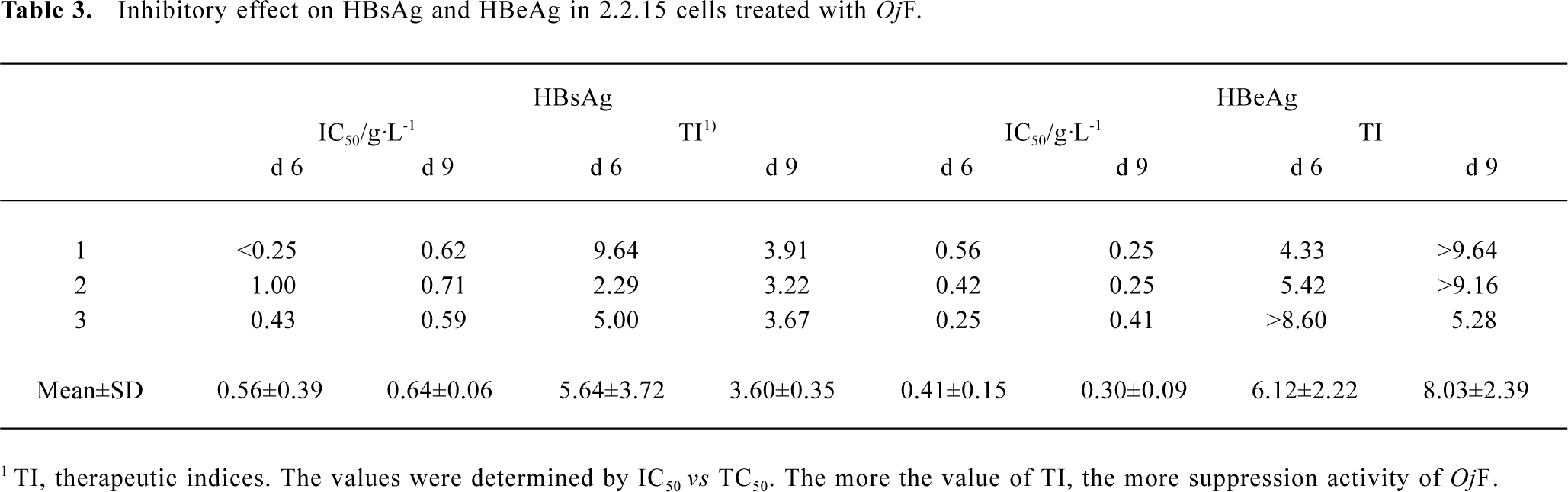
Full table
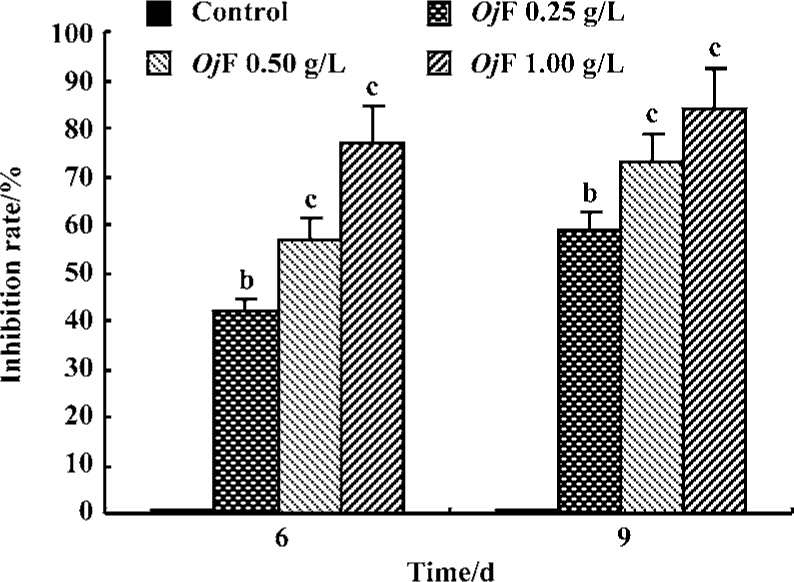
Inhibitory effect of OjF on DHBV-DNA During the course of this study, no obvious side effects were observed in animals receiving antiviral therapy or in control animals.
The effects of OjF and ACV, which were used for comparison, on DHBV replication in vivo were determined by quantification of DHBV-DNA by dot-blot hybridization. The levels of serum viral DNA were recorded in the 5 groups before the experiment. During treatment, serum levels of DHBV-DNA decreased in all 16 ducks treated with OjF 0.50 and 1.00 g·kg-1·d-1 (Table 4). The mean percentage inhibition of viral DNA levels with OjF 0.50 and 1.00 g·kg-1·d-1 was 54.3% and 64.5% respectively on the last day of treatment (Table 5). But 3 days after the cessation of treatment with ACV, the viral replication level returned to the pretreatment baseline. In ducks treated with OjF, the effect of DHBV-DNA inhibition lasted. No significant decrease of serum DHBV-DNA was observed during treatment with OjF 0.25 g·kg-1·d-1. In the control group, serum DHBV-DNA remained unaffected during the course of the study.
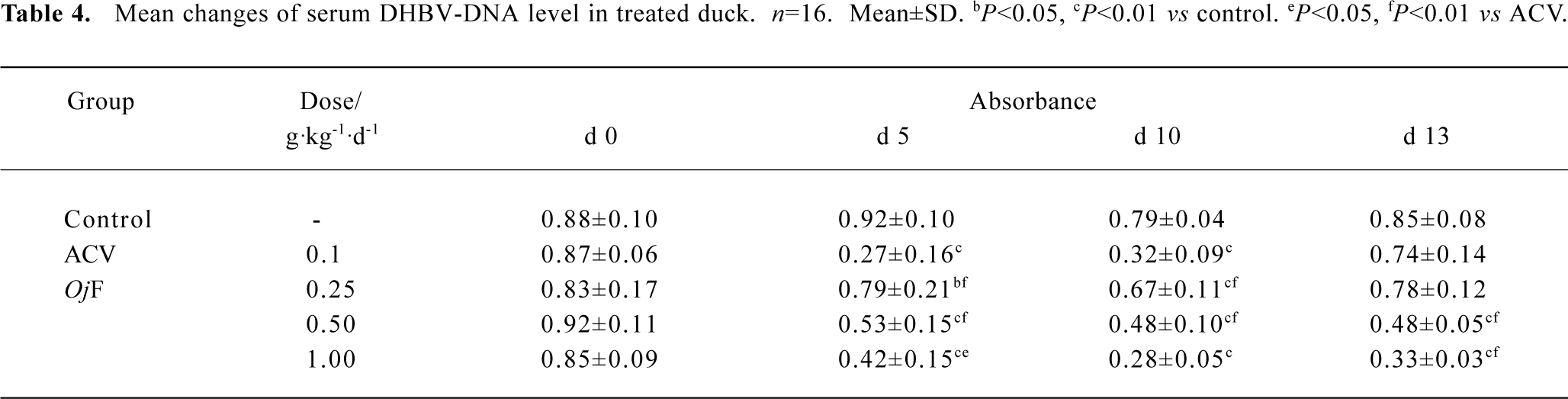
Full table
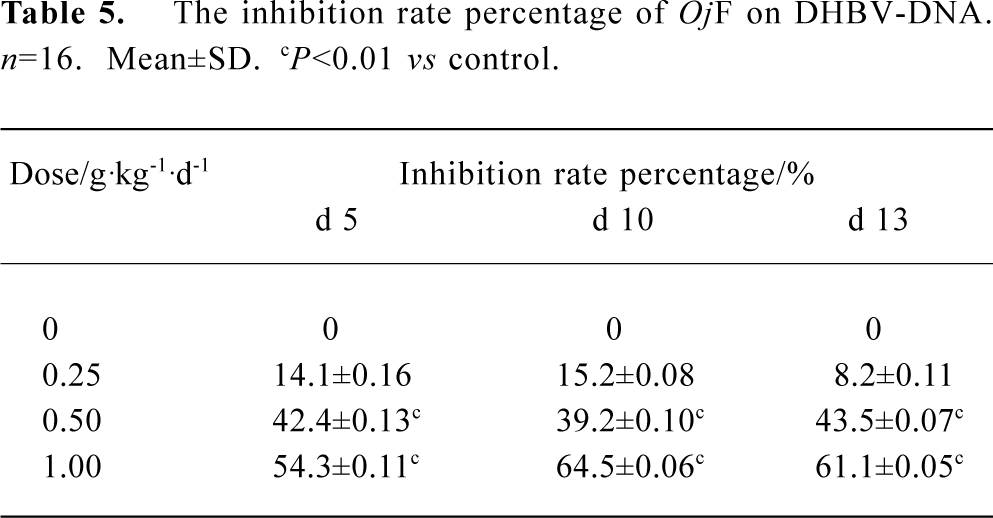
Full table
Histopathological features Histopathological profiles of the liver from model group ducklings revealed necrosis, steatosis, and often swelling of the hepatic cytoplasm. The protective effect of OjF was confirmed by histopathological examinations. Administration of OjF to the experimental animals (1.00 g·kg-1·d-1) showed a significant improvement of the hepatocellular architecture over the model group, as evident from a considerable reduction in necrosis and vacuolation (Figure 3).
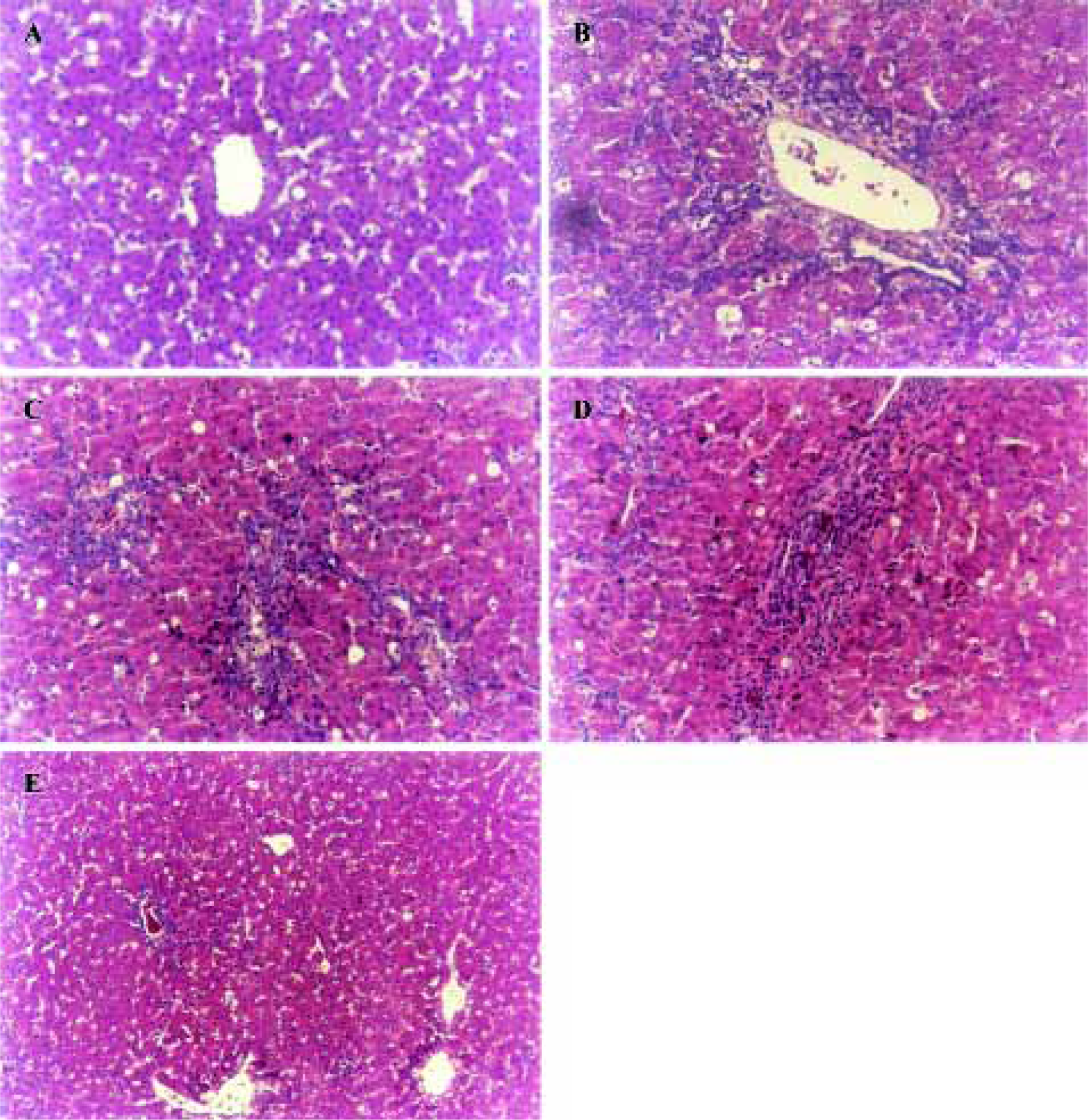
Discussion
Hepatitis B virus causes acute and chronic hepatitis, which affects nearly 360 million people worldwide[12]. Chronic infection with HBV has been associated with a high risk for the development of primary hepatocellular carcinoma[13,14]. Effective antiviral therapy against HBV infection has not been fully developed. Studies have been hampered by the extremely narrow host range and limited access to experimental culture systems. Fortunately, techniques have been developed to propagate hepadnaviridae in tissue culture[15,16] and animal systems[17]. The 2.2.15 cells contain all HBV particles, and the Peking ducks allow multiplication of the HBV-like virus, which make it possible to study the various aspects of the viral life cycle and to examine the effectiveness of potential antiviral drugs.
In a previous study, we have shown that the acid-base extracts of Oj could protect hepatic cells, decrease the content of ALT, AST, and BiliT on a liver damage model caused by carbon tetrachloride (CCl4) in rats[18], and inhibit DHBV[7,8]. Similar results of the inhibitory effect of Oj extracts on HBsAg and HBeAg production in cultured 2.2.15 cells was also observed[19]. However, what the active part of Oj against HBV is is still unknown. OjF was obtained through extraction and separation, which made up more than 50% in the whole extracts. It might be an active part against HBV. Through subsequent purification of OjF, hyperoside, persicarin, isorhamnetin, and quercetin were obtained. Among them, the assay of hyperoside was the highest (purity>96%), which was used as the criterion to control the quality of OjF.
This study demonstrated the inhibitory effect of OjF on HBsAg and HBeAg secretion by human hepatoma 2.2.15 cells and on serum DHBV-DNA levels of ducklings infected with hepatitis B virus. TC50 of OjF was 2.28 g/L and TC0 was 1.00 g/L in 2.2.15 cells, which suggested that the inhibitory action of OjF had no cytotoxicity. In nontoxic concentrations, OjF significantly inhibited the secretion of HBsAg and HBeAg. With OjF concentration increasing, a dose-dependent response was observed. At a TC0 of 1.00 g/L, the inhibition rate percentage of OjF on HBsAg and HBeAg in 2.2.15 cells were both more than 50%, and the inhibition rate percentage on HBsAg exceeded that of OjF on HBeAg.
These results clearly illustrate an inhibitory effect of OjF on HBsAg and HBeAg production in 2.2.15 cells, which provide strong evidence to evaluate the effect of drugs against HBV in a cellular model, but it is still necessary to verify this in an animal model. Therefore, the inhibitory effect of OjF in the duck HBV model was investigated. Our experiments with OjF (0.50 and 1.00 g·kg-1·d-1) in ducklings pointed to a suppressive action on DHBV replication in vivo. With OjF 1.00 g·kg-1·d-1, the therapy caused a more pronounced decrease (64.5%) in viremia. It was well known that most antivirus medicines had the inevitable rebound effect after drug cessation. This shortcoming had limited the therapy to those diseases infected by viruses such as HB or AIDS. The similar phenomena appeared in the positive control drug ACV in the present study. OjF showed therapeutic effects as well as ACV, and no difference was observed after cessation of OjF therapy compared to OjF-treated animals. It suggested that OjF could maintain for a long time in treating viremia of HBV and the effect of DHBV-DNA inhibition showed a concentration-dependent response. Histopathological examination also confirmed the function of OjF protecting the liver in DHBV-infected ducklings. Xiong Q et al[19] reported that Apocynum ventun extracts containing hyperoside and quercetin had hepatoprotective activity, and OjF contained these two compounds. These results demonstrated the antihepatitis B virus effect of OjF, which were consistent with antiviral activity of Oj[7,8], making OjF a candidate for future evaluation in patients with HBV infection.
In order to elucidate the possible mechanism of OjF towards DHBV-DNA on DHBV-infected ducklings, the effect of OjF on DHBV-DNA was investigated (data not shown). Our results indicate that OjF might inhibit the DNA-dependent DNA polymerase reaction, which results in the termination of replication of DHBV-DNA. This hypothesis is currently under investigation in our laboratory.
In conclusion, OjF possessed the significant antiviral activity in vitro and in vivo, and it was one of the main active parts of Oj against HBV. Elucidation of the mechanism of its antiviral activity and identification of the active components in OjF will greatly enhance the understanding of viral gene expressions and provide new clues to assist in the development antiviral agents in the future.
Acknowledgement
The authors wish to thank Zhuang LI for his excellent technical support in this study. This research was supported by Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences.
References
- Huang ZM, Yang XB, Cao WB. Modern study and clinic application of Oenanthe javanica. Pharm J Chin PLA 2001;17:266-9.
- Huang ZM, Zhang ZM, Yang XB, Cao WB. Study of Shui Qin on antihepatitis. Pharmacol Clin Chin Mat Med 1991;7:11-3.
- Yang XB, Huang ZM, Cao WB. Effect of an aqueous extract from Oenanthe javanica on rat cardiovascular system. Chin Tradit Herb Drugs 1998;10:47-9.
- Ji GJ, Cao WB, Huang ZM. Anti-arrhythmic effect of injections of SQ. Chin Pharm J 1990;15:134-8.
- Zhang JZ, Cao WB, Yang XB, Huang ZM. Anti-anaphylactic effect of the decoction of SQ. Pharmacol Clin Chin Mat Med 1992;8 suppl:29-32.
- Yang XB, Huang ZM, Cao WB. Antidiabetic effect of Oenanthe javanica flavone. Acta Pharmacol Sin 2000;21:239-42.
- Huang ZM, Yang XB, Cao WB. Effects of Qin ling ke li in the treatment of 90 patients with chronic hepatitis B. Pharm J Chin PLA 2001;17:41-4.
- Huang ZM, Yang XB, Cao WB. Inhibition of shuiqin on DHBV in vitro. Chin Pharm J 1997;32:720-3.
- Huang ZM, Yang XB, Cao WB. Study of inhibition of duck hepatitis B virus by extract from Oenanthe javanica. Chin Sci Bull 1997;42:1863-7.
- Sha M, Cao A, Wang B. Determination of Hyperin in Sanguisorba officinalis L by high performance liquid chromatography. Chin J Chromatogr 1998;16:226-8.
- Wu Y, Zhou SD, Li P. Determination of flavonoids in Hypericum perforatum by HPLC analysis. Acta Pharm Sin 2002;37:280-2.
- Lamivudine Clinical Practice Group. Lamivudine treatment consensus from relative experts in 2004. Chin J Hepatol 2004;12:425-7.
- Hantz O, Perigaud C, Borel C, Jamard C, Zoulim F. The SATE pronucleotide approach applied to acyclovir Part II. Effect of bis(SATE)phosphotriester derivatives of acyclovir on duck hepatitis B virus replication in vitro and in vivo. Antiviral Res 1999;40:179-87.
- Liu J, Shen HM, Ong CN. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG2 cells. Cancer Lett 2000;153:85-93.
- Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in HepG2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA 1987;84:1005-9.
- Wu B, Wang CN, Xu JR. Effect of epidermal growth factor on cultured rat hepatocytes poisoned by CCl4. Acta Pharmacol Sin 1997;18:176-9.
- Liu J, Liu YP, Curtis DK. Protective effect of oleanolic acid against chemical-induced acute necrotic liver injury in mice. Acta Pharmacol Sin 1995;16:97-102.
- Chen HY, Liu J, Zhu HF, Xu L. Action of acid-base extract of Oenanthe javanica on mice models of CCl4 hepatic injury. Pharm J Chin PLA 2001;17:22-4.
- Xiong QB, Fan WZ, Tezuka YH. Hepatoprotective effect of Apocynum ventun and its active constituents. Planta Med 2000;66:127-33.
