Expression of pro-inflammatory and anti-inflammatory cytokines in brain of atherosclerotic rats and effects of Ginkgo biloba extract1
Introduction
Atherosclerosis (AS) is not merely a disease in its own right, but a process that is the principal contributor to the pathogenesis of cardiovascular and cerebrovascular diseases. Despite the universal occurrence of atherosclerosis in the world, the pathogenesis of disease remains incompletely understood. In the injury theory of Ross, atherosclerosis can be considered to be a modified form of chronic inflammation. Uptake of oxidized low density lipoprotein (ox-LDL) by the macrophages through scavenger receptor (SR) will lead to foam cells formation and numerous cytokines; cell adhesion molecules, chemotactic factor and growth factors were excreted. All these factors induce the migration and proliferation of smooth muscle cells (SMC), this process repeats until the formation of atherosclerotic plaque. In recent years research results have indicated that an anti-inflammatory cytokine such as IL-10 is also concerned in the generation and development of AS, and can play an important protective role[1].
The rat was considered suitable for establishing cardiovascular disease model including cardiac hypertrophy, hypertension, and heart failure; but it is not such a good model in atherosclerosis due to its hyperlipidemia-resistant property. Recently, our task-group established the experimental model of AS in rats by combining the high fat/cholesterol diets containing sodium cholate and propyl-thyracil with injection of vitamin D3[2].
Ginkgo biloba extract (GbE) has beneficial effects on cardiovascular disease. Our previous study showed that the inhibitory effects of GbE on vascular endothelial growth factor-induced permeability of bovine coronary endothelial cells indicated that GbE had the potential to protect against atherosclerosis[3].
Up to now, whether the expression of anti-inflammatory cytokines will have a change in the brain of rat with atherosclerosis remains unknown. The present study aims to investigate whether the anti-AS effect of GbE was related with the expression of pro-inflammatory cytokines such as IL-1β and TNF-α, and anti-inflammatory cytokines such as IL-10 and IL-10R in the brain.
Materials and methods
Reagents GbE (containing 24% flavone glycosides and 6% gingko lactone) was presented by Prof Wei-zhou CHEN (Shanghai Institute of Material Medica, Shanghai, China). Rat IL-1β, TNF-α, and IL-10 ELISA kit were purchased from Biosource (Camarillo, California, USA); Tissue/Cell Total RNA Isolation Kit was purchased from Watson Co (Shanghai, China); TaKaRa RNA PCR kit (AMV) Ver 2.1 was purchased from TaKaRa Biotechnology Co (Dalian, China). The antibodies for IL-1β, TNF-α, and IL-10 were purchased from Santa Cruz Biotechnology, Inc (Santa Cruze, CA, USA). Streptavidin/peroxidase (SP) kit and diaminobenzidine (DAB) kit were purchased from Zhongshan Biotechnology Co (Beijing, China).
Experimental protocol Thirty male Wistar rats were supplied by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences. Qualification number is SCXK (hu) 2002-0010. The AS rat model was established by intra-peritoneal injection with a single dose of vitamin D3 600 kU/kg in addition to loading with high fat/cholesterol diet containing sodium cholate and propyl-thyracil as described previously[2]. The rats were divided into three groups: control, AS, and GbE. The control group was fed with ordinary forage, while the AS and GbE groups were fed with high fat/cholesterol diet after injection of vitamin D3. At the same time, GbE group rats were administered GbE 100 mg/kg through intra-gastric method every day for 8 weeks.
Enzyme-linked immunosorbant assay (ELISA) The rat brains were homogenated with nomal saline at 2:1 ratio. The supernatant was used for the measurement of cytokine protein by ELISA as described in kit specification.
Immunohistochemistry The brain tissue was fixed in 4% paraformaldehyde and embedded in paraffin. Immunohistochemistry analysis was performed as specified by the kit protocol. Rabbit normal immune serum was used to replace primary antibody in the negative control group. PBS was used to replace immediate antibody in the control group.
Western blotting was performed to detect the cytokine protein expressions. Rat brain tissues were cut into small pieces. Protein extracts were prepared by homogenizing the 1 g brain tissues in cold 3 mL radioimmunoprecipitation (RIPA) buffer with a glass tissue homogenizer. The tissue homogenates were centrifuged at 10 000×g 4 °C for 10 min. The supernatants were collected and centrifuged at 10 000×g 4 °C for 10 min again. Protein concentration in the supernatant was determined by BCA method. Two times of loading buffer was then added to each supernatant with equal volume containing equal amounts of protein, which was subsequently boiled for 5 min and then electrophoresed on a 10% SDS-polyacrylamide gel. Proteins were transferred to nitrocellulose membrane and incubated sequentially with antibodies, and then with peroxidase-conjugated secondary antibodies in the second reaction. Detection was performed with enhanced chemiluminescence reagent.
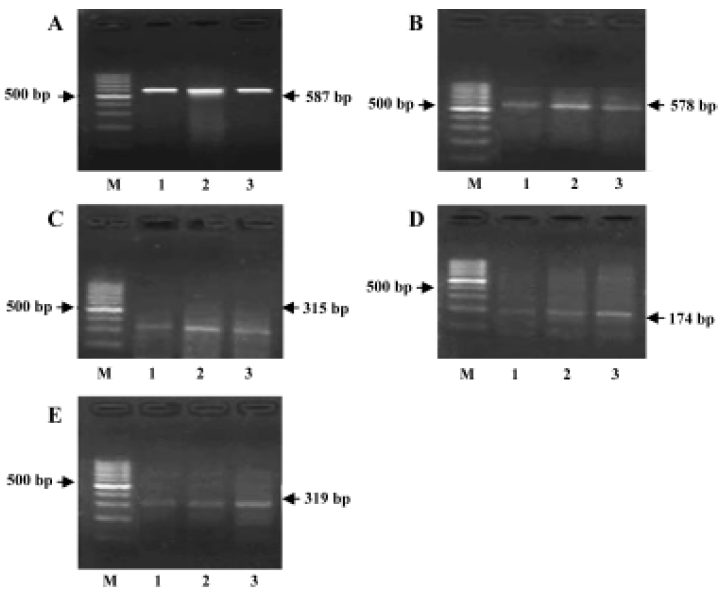
RT-PCR for detection of the cytokine mRNA expressions Total RNA was isolated by Tissue/Cell Total RNA Isolation Kit. Total RNA was quantified with the ratio of absorption values of RNA samples at 260 nm and 280 nm. RT-PCR was performed by RNA PCR kit. Beta-actin was used as an internal positive control. Specific primer sequences of beta-actin, IL-1β[4], TNF-α, IL-10[5], and IL-10R[6] were as follows and the sizes of production were 587, 578, 315, 174, and 319 bp, respectively:
β-actin: sense 5'- AAGGCCAACCGTG AAAAGATGAC-3'; antisense 5'-GGGTACATGGTGGTGCCACCAGAC -3';
IL-1β: sense 5'-GACCTGTTCTTTGAGGCTGAC-3'; antisense 5'- TCCATCTTCTTCTTTGGGTATTGTT-3';
TNF-α: sense 5'-CATGATCCGAGATGTGGAACTGGC-3'; antisense 5' -CTGGCTCAGCCACTCCAGC -3';
IL-10: sense 5'- TAAGGGTTACTTGGGTTGCCAAGCC -3'; antisense 5'-AGGGGAGAAATCGATGACAGCG-3';
IL-10R: sense 5'- CCAACTGGACCATCACTGAAACTC -3'; antisense 5'- GCCTTGTTAATTCGGGATTCCAC- 3'.
The temperature profile of the amplification consisted of 45-s denaturation at 94 °C, 45-s annealing at 50 °C, and 2-min extension at 72 °C at 35 cycles. PCR products were sequenced to verify that desired product was amplified.
Statistical analysis Data were presented as mean±SD. Statistical significance was assessed by ANOVA test. P<0.05 was considered statistically significant.
Results
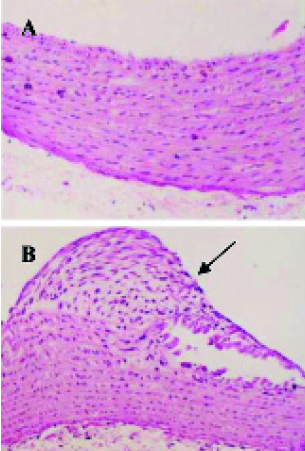
The experimental model of AS in rat was established (Figure 1).
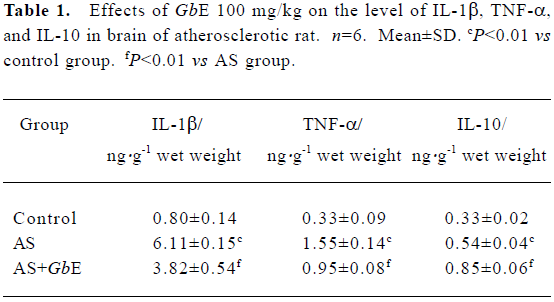
IL-1β, TNF-α, and IL-10 protein levels in brain of AS rat by ELISA The protein levels of IL-1β, TNF-α, and IL-10 in the brains of the AS group were significantly increased compared with the control group. GbE 100 mg/kg inhibited the protein levels of IL-1β and TNF-α, but up-regulated the protein levels of IL-10 (Table 1).
Full table
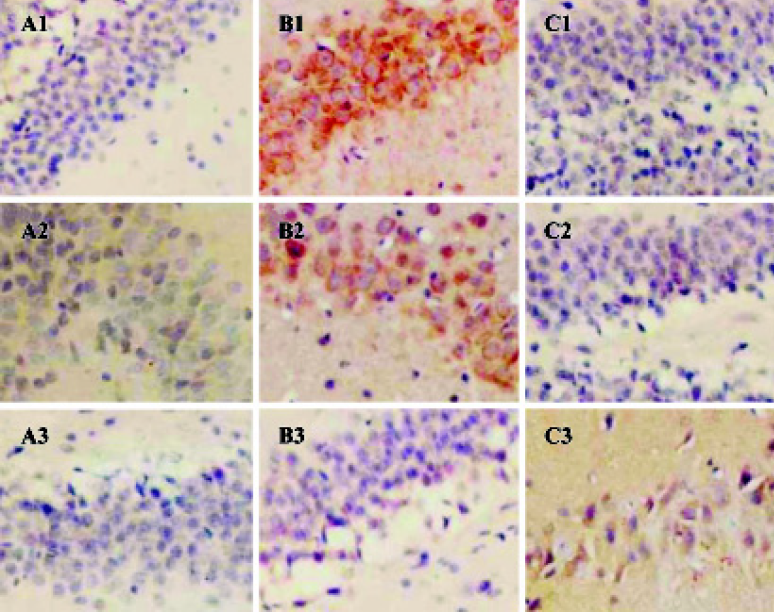
IL-1β, TNF-α, and IL-10 protein expressions in brain of AS rat Immunohistochemistry analysis showed that the expressions of IL-1β, TNF-α, and IL-10 were significantly increased in the cerebral cortex, hippocampus, and hypothalamus of AS rats compared with the control group. After the AS rats were given GbE 100 mg/kg ig, the expressions of IL-1β and TNF-α in brain were decreased in the cerebral cortex, hippocampus, and hypothalamus but the expression of IL-10 was higher than pretreatment (Figure 2).
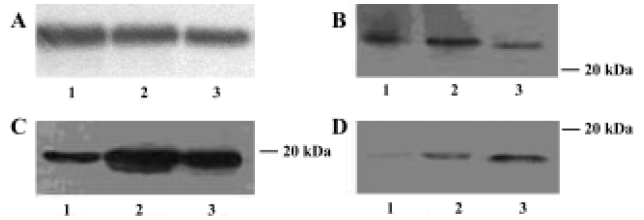
IL-1β, TNF-α, and IL-10 protein expressions in brains of AS rats Western blotting analysis indicated that in the brains of the control group IL-1β and TNF-α were both expressed, and IL-10 had nearly no expression. The expressions of IL-1β, TNF-α, and IL-10 protein were all significantly increased in brain of AS rats compared with the control group. The expressions of IL-1β and TNF-α in brains were markedly lower in the GbE group than that in AS group; but the expression of IL-10 in brains were markedly higher in the GbE group than that in the AS group (Figure 3).
IL-1β, TNF-α, IL-10, and IL-10R mRNA expressions in the brains of AS rats RT-PCR analysis showed that IL-1β, TNF-α, IL-10, and IL-10R mRNA were all seldom expressed in the brains of the control group. The expressions of IL-1β, TNF-α, IL-10, and IL-10R mRNA were all markedly increased in the brains of AS rats compared with the control group. The expressions of IL-1β and TNF-α were lower, but the expressions of IL-10 and IL-10R mRNA were higher in the GbE group than that in the AS group (Figure 4).
Discussion
We observed that the expressions of IL-1β, TNF-α, Il-10, and IL-10R in the brain were markedly higher in the AS rats than that in the control. The mRNA and protein expressions of IL-1β and TNF-α in the brains were markedly lower, but the mRNA and protein expressions of IL-10 and IL-10R in the brains were markedly higher in the GbE 100 mg/kg group than that in the AS group. Our results were consistent with the recent findings that the serum levels of TNF-α and IL-1β in all patients with coronary heart diseases were higher than those in controls[7–9].
IL-10 was an anti-inflammatory cytokine with powerful immunoregulation potential[10]. It was excreted from monocyte/macrophages and smooth muscle cells under certain conditions. Mallat et al found that IL-10-deficient mice fed with an atherogenic diet and raised under specific pathogen-free conditions exhibited a significant three-fold increase in lipid accumulation compared with wild-type mice[11]. Interestingly, the susceptibility of IL-10-deficient mice to atherosclerosis was more higher (30-fold increase) when the mice were housed under conventional conditions. Atherosclerotic lesions of IL-10-deficient mice showed increased T-cell infiltration, abundant interferon-γ expression, and decreased collagen content. In vivo, transfer of murine IL-10 achieved a 60% reduction in lesion size. These results indicated the critical roles of IL-10 in both atherosclerotic lesion formation and its stability. Serum levels of the anti-inflammatory cytokine, interleukin-10, are decreased in patients with unstable angina[12], this important finding also suggests that IL-10 has a protective role in atherosclerosis.
In other countries GbE was used to treat cardiovascular and cerebrovascular diseases such as coronary heart diseases and stroke[13–18]. Our findings showed that GbE inhibited the mRNA and protein expression of pro-inflammatory cytokines IL-1β and TNF-α, and up-regulated the mRNA and protein expression of anti-inflammatory cytokines, IL-10 and IL-10R in the brain of AS rats, which might be related with its anti-AS effects.
References
- Mallat Z, Heymes C, Ohan J, Faggin E, Leseche G, Tedgui A. Expression of interleukin-10 in advanced human atherosclerotic plaques: relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol 1999;19:611-6.
- Huang ZY, Yang PY, Almofti MR, Yu YL, Rui YC, Yang PY. Comparative analysis of the proteome of left ventricular heart of arteriosclerosis in rat. Life Sci 2004;75:3103-15.
- Qiu Y, Rui YC, Li TJ, Zhang L, Yao PY. Inhibitory effect of extracts of Ginkgo biloba leaves on VEGF-induced hyperpermea-bility of bovine coronary endothelial cells in vitro. Acta Pharmacol Sin 2004;25:1306-11.
- Hansen MK, Taishi P, Chen Z, Krueger JM. Cafeteria feeding induces interleukin-1β mRNA expression in rat liver and brain. Am J Physiol 1998;274:R1734-9.
- Adams JK, Tepperman BL. Colonic production and expression of IL-4, IL-6, and IL-10 in neonatal suckling rats after LPS challenge. Am J Physiol Gastrointest Liver Physiol 2001;280:G755-62.
- Mathurin P, Xiong S, Kharbanda KK, Veal N, Miyahara T, Motomura K, et al. IL-10 receptor and coreceptor expression in quiescent and activated hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2002;282:G981-90.
- de Maat MP, Kluft C. The association between inflammation markers, coronary artery disease and smoking. Vascul Pharmacol 2002;39:137-9.
- Wang YN, Che SM, Ma AQ. Clinical significance of serum cytokines IL-1β, sIL-2R, IL-6, TNF-α, and IFN-γ in acute coronary syndrome. Chin Med Sci J 2004;19:120-4.
- Balbay Y, Tikiz H, Baptiste RJ, Ayaz S, Sasmaz H, Korkmaz S. Circulating interleukin-1 beta, interleukin-6, tumor necrosis factor-alpha, and soluble ICAM-1 in patients with chronic stable angina and myocardial infarction. Angiology 2001;52:109-14.
- Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest 2000;117:1162-72.
- Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res 1999;85:17-24.
- Smith DA, Irving SD, Sheldon J, Cole D, Kaski JC. Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation 2001;104:746-9.
- Kleijnen J, Knipschild P. Ginkgo biloba. Lancet 1992;340:1136-9.
- Lin JY, Cheng FC, Chung SY, Lin MC. Ginkgo biloba extract (EGb761) and FK506 preserve energy metabolites in the striatum during focal cerebral ischemia and reperfusion in gerbils monitored by microdialysis. J Biomed Sci 2004;11:611-6.
- Sun BL, Zhang J, Wang XC, Xia ZL, Yang MF, Zhang SM, et al. Effects of extract of Ginkgo biloba on spasms of the basilar artery and cerebral microcirculatory perfusion in rats with subarachnoid hemorrhage. Clin Hemorheol Microcirc 2003;29:231-8.
- Chung SY, Wang MF, Lin JY, Lin MC, Liu HM, Cheng FC. Effect of one week treatment with Ginkgo biloba extract (EGb761) on ischemia-induced infarct volume in gerbils. Am J Chin Med 2003;31:533-42.
- Guillon JM, Rochette L, Baranes J. Effects of Ginkgo biloba extract on 2 models of experimental myocardial ischemia. Presse Med 1986;15:1516-9.
- Chen J, Wang X, Zhu J, Shang Y, Guo X, Sun J. Effects of Ginkgo biloba extract on number and activity of endothelial progenitor cells from peripheral blood. J Cardiovasc Pharmacol 2004;43:347-52.