Development of Caenorhabditis elegans pharynx, with emphasis on its nervous system
Introduction
Caenorhabditis elegans (C elegans) is a 1-mm long free-living nematode that currently has tremendous popularity as a model organism, especially regarding questions of interest to developmental biologists. Given its ease of culture (it is typically grown on Escherichia coli lawns), a 3-d life cycle from egg to egg, a transparent body that allows visualization of any cell of interest, a fully described cell lineage, and the ease with which genetic screens can be carried out at low costs, it is no wonder that C elegans is such a versatile and popular laboratory model organism. The 97 Mb genome of C elegans is also completely sequenced, and computer algorithms predict nearly 20 000 functional genes. Furthermore, the C elegans research community has a traditional helpfulness and openness: thousands of mutants are freely available to academic laboratories from the C elegans Genetics Center (http://biosci.umn.edu/CGC/CGChomepage.htm), and public databases of anatomical and molecular information are available on the internet (http://www.wormatlas.org/; http://www.wormbase.org/).
Here, we review the developmental genetics of the C elegans pharynx, with an emphasis on the development of its small 20-neuron network. However, here is one last introductory note: several lines of evidence suggest that the C elegans pharynx evolved from an organ that was also the common ancestor to the vertebrate heart. This evidence consists mostly of physiological and molecular similarities: (i) like the heart, the pharynx is a rhythmically contracting neuromuscular pump[1]; (ii) the muscle cells of the pharynx have autonomous contractile activity reminiscent of cardiac myocytes[2]; and (iii) ceh-22, the C elegans homolog to the homeobox gene NK2.5 that plays an important role in heart development in vertebrates, participates in pharyngeal development, and can partially be replaced functionally by the zebrafish NK2.5[3]. The evolutionary relatedness of the C elegans pharynx and the vertebrate heart suggest that insights regarding heart development and function may be gained by studying the simpler and experimentally more convenient C elegans pharynx.
Pharynx form and function
The pharynx represents the foregut of the nematode digestive tract (Figure 1). Food (typically E coli in the labora-tory) is pumped through the mouth by the action of the muscular pharynx, ground by specialized cuticle lining the pharynx (the “grinder” in the posterior bulb), and transferred to the intestine via a pharyngeal-intestinal valve. The main anatomical features of the pharynx are, from anterior to posterior, the procorpus, the metacorpus, the isthmus, and the posterior bulb in which the grinder is located (Figure 1). The mature pharynx is composed of 62 cells (for a total of 80 nuclei, since several of the cells are binucleate as a result of cell fusion). These cells can be categorized into 5 types: neurons (20), muscles (20 cells; 37 nuclei), marginal cells (9), epithelial cells (9), and gland cells (4 cells; 5 nuclei). The muscle cells and marginal cells constitute a single-cell-thick tube, continuous at its anterior end with the tube of the hypodermis that encloses the worm. Muscle and marginal cells are joined by tight junctions, which divide the membrane into apical and basal surfaces. The apical surfaces face the lumen and secrete cuticle, continuous with the cuticle made by the hypodermis. The basal surfaces face a basal lamina that is continuous with the basal lamina that separates the hypodermis and intestine from the pseudo-coelom (fluid-filled body cavity) and mesoderm. Components of this basal lamina are likely produced by body-wall muscles[4,5]. The 9 epithelial cells are arranged so as to form a narrow ring at the anterior end of the pharynx, where it connects with the buccal cavity. There is otherwise no epithelial sheet covering the bulk of the pharynx. Precise knowledge of pharyngeal anatomy is available at the ultrastructural level, thanks to detailed electron microscopy studies[6].
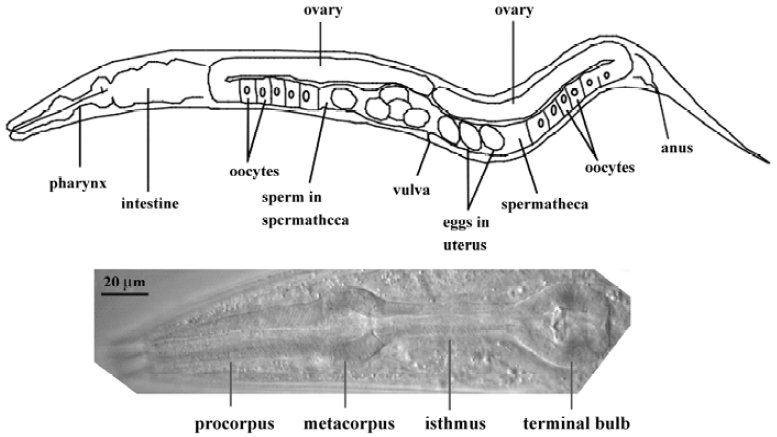
Pharyngeal neurons lie deep within folds of the basal membrane of pharyngeal muscle cells (note that this is not a “basal lamina” or “basement membrane”, but is that part of the muscle cell membranes that is on the “basal” side), between the muscle and basal lamina, just as the extrapha-ryngeal nervous system is between the basal membrane of the hypodermis and the basal lamina. No basal lamina separates pharyngeal motor neuron presynaptic terminals from the post-synaptic muscle membrane. In contrast, extrapha-ryngeal motor neurons are separated from the muscle cells on which they synapse by the basal lamina that separates the mesodermal muscle cells from the ectodermal neurons.
The role of the pharyngeal nervous system in regulating pumping is somewhat of a mystery. Normal feeding consists of two primary motions: pumping and isthmus peristalsis[1]. A pump is a near-simultaneous contraction of the muscles of the corpus, anterior isthmus, and terminal bulb, followed by a near-simultaneous relaxation. The contractile fibers of the pharyngeal muscles are radially oriented, so contraction pulls the lumen open from its resting closed Y-shape to a triangular shape. The second motion, isthmus peristalsis, occurs after the main relaxation is complete. It is a peristaltic wave of contraction in the posterior isthmus that carries bacteria trapped in the anterior isthmus back to the grinder. Typically, only every fourth pump is followed by an isthmus peristalsis. The nervous system is not essential for pumping; pumping continues even when the entire pharyngeal nervous system is killed[2]. However, many neurons are important; efficient pumping and trapping of bacteria by the pharynx requires the presence of the neurons I5, MC, M3, M4, and NSM[2,7,8].
Development of the pharynx
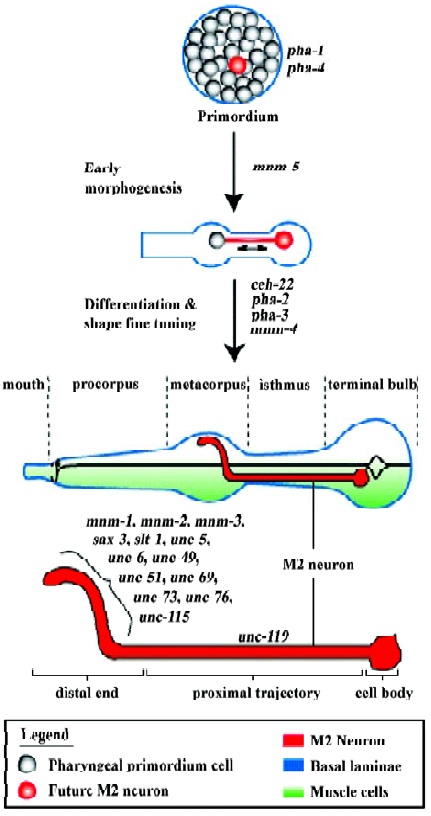
In order to begin understanding how the pharyngeal neurons develop, it is necessary first to describe pharyngeal development itself (Figure 2). The C elegans pharynx offers a very simple model to understand morphogenesis and differentiation. The pharynx develops through the morphogenesis of a primordium composed of 80 undifferentiated cells (plus many apoptotic cells; there are 19 apoptotic cells that are sisters to final pharyngeal cells and that die within 350– 420 min of development[9]). Morphogenesis is accompanied by differentiation but not by new cell divisions, so the mature pharynx contains 80 nuclei but only 62 cells as a result of cell fusion among some of the muscle and two gland cells; these fusions occur around the time of hatching and seem irrelevant to the developmental process[9], although it would be interesting to understand how these fusions are regulated.
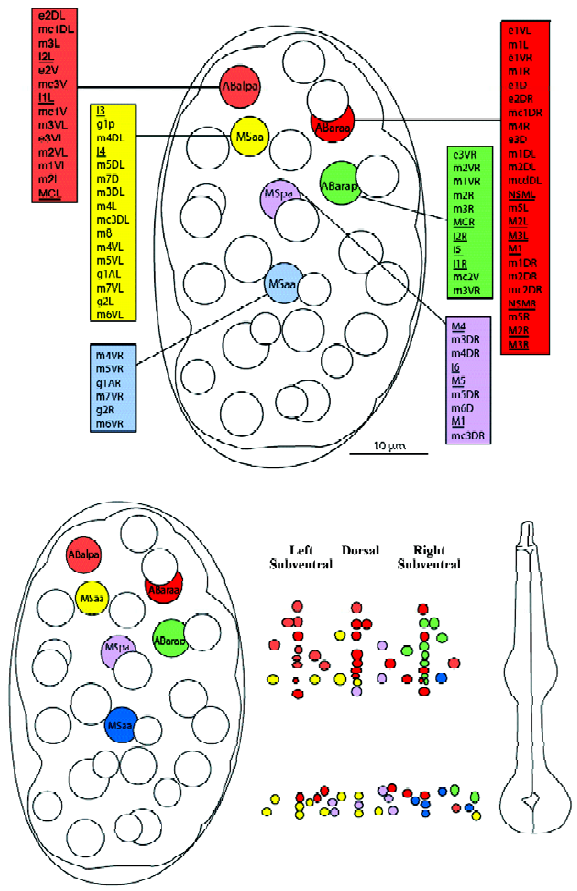
0–100 min: early cell divisions and establishment of main lineages The cells that make up the pharyngeal primordium originate from two early embryonic blastomeres: the ABa and the MS blastomeres. This is quite remarkable: members of two distinct lineages are recruited to form one organ. Not only that, but cells with these two very different ancestries may end up adopting nearly identical fates. For example, the muscle cell m3VL has the ancestry ABalpappppp, whereas the identical cell m3DL has the ancestry Msaaapaaa (these two cells will fuse later). Note that even though each cells is normally specific to adopt a developmental fate, there is some degree of developmental plasticity. For example, Avery and Horvitz showed that the pharyngeal neuron M4 is essential for feeding in wild-type C elegans, but that in a ced-3 mutant (in which the sister cell of M4 does not die of apoptosis), the now viable sister of M4 can sometimes take over the function of M4[10].
The respective contributions of the ABa and MS lineages are more or less spatially consistent with their initial positions within the 8-cell embryo. For example, the anterior cell ABa contributes cells of the anterior pharynx, whereas the more posterior MS cell contributes mostly posterior pharyngeal cells. This observation holds true for later descendents and narrower scopes of spatial contributions. Figure 3 shows the adult pharyngeal contributions from the pharyngeal precursors of the 100-cell stage embryo, and emphasizes the preservation of spatial relationships during development. Thus, ABalpa contributes mostly to the anterior left subventral area, etc.
100–250 min: gastrulation At 100 min after first cleavage, when the egg comprises 28 cells, gastrulation begins. During gastrulation, several cells enter deep into the embryo through a ventral cleft. The first cells to enter are the gut precursor cells Ea and Ep. Next are the P4 and MS progeny at 120–200 min of development, and the AB-derived pharyngeal precursors enter more anteriorly at 210–250 min. The ventral cleft closes from posterior (230 min) to anterior (290 min). As gastrulation proceeds, the E cell descendents and the pharyngeal precursors form a central cylinder. Note that as gastrulation proceeds, so do cell divisions. Active pre-pharyngeal cell divisions continue until approximately 350 min of development, and some late divisions occur until approximately 400 min.
250–400 min: compaction of pharyngeal primordium Between 250 min and 400 min the pharyngeal primordium becomes clearly defined. The non-pharyngeal precursor cells are somehow excluded from the pharyngeal primordium. Perhaps they are squeezed out in a process by which the pharyngeal cells have more adhesive affinity to each other than to any other cell (in line with the theory of Malcolm Steinberg; eg see Duguay et al[11]). This aspect of primordium formation has not been investigated experimentally.
400–430 min: extension of pharyngeal primordium The approximately 400-min-old primordium is insulated by a basement membrane (present at or before 400 min[9]), such that the pharynx develops autonomously, perhaps with no extrapharyngeal cues, or with very few. Such autonomous development is also true of the 20-cell intestine that, together with the pharynx, makes up the entire C elegans gut[12]. At approximately 400 min, the pharyngeal primordium is approximately spherical, and most of the cell nuclei appear located in spatial relationships that are consistent with their final positions, at least along the anterior-posterior axis, although the relative distances between these nuclei can be very different from those of the mature organ. For example, at approximately 430 min, the sister cells M2 and M3 have their nuclei next to each other, whereas in the final pharynx M2 has its nucleus in the posterior bulb and M3 in the meta-corpus. It therefore seems that development of the pharynx is mostly a question of cell differentiation and morpho-genesis, not of active cell migration. However, some cells do migrate within the developing pharynx. For example, Sulston et al observed that the 3 g1 gland cells migrate in a reproducible way. They wrote: “Their movements approximately follow the subsequent course of their secretory processes, and may be responsible for laying down the latter”[9].
Beginning at approximately 400 min, the primordium elongates anteriorly then posteriorly. The primordium develops into a tube connected anteriorly to the buccal cavity and posteriorly to the midgut. The adherens junctions that connect many pharyngeal cells with each other form simultaneously with the process of elongation. Note that there is no evidence of any basement membrane within the elongated pharynx during or after elongation or at any other stage of development or adulthood[5]. Portereiko and Mango have studied the morphogenesis of the pharyngeal primordium and divided the process into three stages: (i) lengthening of the nascent pharyngeal lumen by reorientation of the apicobasal polarity of anterior pharyngeal cells (“Reorientation”); (ii) formation of an epithelium by the buccal cavity cells, which mechanically couples the buccal cavity to the pharynx and anterior epidermis (“Epithelialization”); and (iii) a concomitant movement of the pharynx anteriorly and the epidermis of the mouth posteriorly to bring the pharynx, buccal cavity, and mouth into close apposition (“Contraction”)[4].
430–800 min: completion of functional pharynx Between 430 min and 490 min, as elongation proceeds, the pharyngeal bulbs and isthmus become apparent. It is probably at this time that the pharyngeal cells interpret their final differentiation programs and adopt their final shapes. Between 600 min and 650 min, the pharyngeal cuticle is produced and the lumen becomes distinct. The pharyngeal glands are active by 720 min and the pharynx is pumping by 750 min. Hatching occurs at approximately 800 min following first cleavage.
Genetics of pharyngeal development
What follows is a brief overview of some of the genes that have been shown to play a role in pharyngeal develop-ment.
pha-4 pha-4 encodes the C elegans homolog of FoxA, a fork-head transcription factor[13]. The pha-4 gene is expressed in all pharyngeal cells, and also in some cells of the rectum[13]. Expression of PHA-4 is detected in all pharyngeal precursor cells beginning from at least 200 min of development (and perhaps even earlier). By the comma stage (~430 min), all the pharyngeal cells are present and express PHA-4. PHA-4 is also expressed in the 6 cells of the pharyngeal intestinal valve, which is not considered a part of the pharynx per se. At 430 min, PHA-4 expression is also found in 6–8 rectal cells, including the 2 rectal valve cells and the 3 rectal epithelial cells. This expression pattern is therefore conserved with that of the Drosophila forkhead gene (high levels in the foregut/pharynx and hindgut/rectum). The pha-4 mutants completely lack all pharyngeal cells, even though the AB and MS lineages are otherwise completely normal[14]. It seems that pha-4 acts as an organ identity factor. Indeed, Gaudet and Mango have proposed that the PHA-4 protein may directly activate most or all pharyngeal genes, with the expression timing being regulated by the presence of binding sites of varying affinity: poor binding sites will have delayed expression, as they will require higher levels of PHA-4 before becoming activated[15]. The consensus binding site for PHA-4 has been defined as: TRTTKRY (R=A/G, K=T/G, Y=T/C). This site is present in the myo-2 gene, a pharyngeal-specific muscle myosin that is a confirmed direct target of PHA-4[13]. Ectopic expression of PHA-4 causes ectopic expression of myo-2, ceh-22 (a homeodomain protein that is also a coactivator of the myo-2 gene), pha-2 (another homeodomain protein important for pharynx development, see below), and most likely other otherwise pharyngeal specific genes[13,16].
pha-1 In pha-1 mutants, the pharyngeal primordium appears to form normally, with a full complement of nuclei and surrounded by a basal membrane. In these mutants, elongation also appears normal up to at least 420 min of development, including the expression of an antigenic marker for the pharyngeal muscle cell precursors detected with the monoclonal antibody 3NB12[17,18]. After elongating and contacting the buccal cavity, the developing organ detaches from the buccal cavity and retracts, causing a “Pun” (pharynx unattached) phenotype. The end result is a worm in which the incompletely formed pharynx is slightly elongated, surrounded by the visible basement membrane and unattached to the mouth. It is difficult to determine if all pharyngeal cell differentiation events take place in the pha-1 mutant, but expression of MYO-2::GFP is detected and a pharyngeal lumen forms[17]. Thus pha-1 affects pharynx development after pharynx cells are committed to a specific cell fate, but before terminal differentiation/morphogenesis of the different pharyngeal cell types occurs[17,18].
Initial analysis of the PHA-1 amino acid sequence suggested that it was a basic leucine zipper (bZIP) transcription factor. Expression of a PHA-1::LacZ reporter also suggested restricted expression in pharyngeal cells as well as in body muscle cells[18]. However, a more recent evaluation of the PHA-1 amino acid sequence indicates that pha-1 actually does not encode a bZIP transcription factor[17]. Consistent with this last analysis, a rescue-competent PHA-1::GFP fusion protein suggests that PHA-1 is a cytosolic protein[17] that is widely expressed (essentially in all cells by the 100-cell stage). Because the biochemical function of PHA-1 is unknown at present, little can be said about its actual mechanism of action. However, genetic interaction experiments have shown that pha-1, lin-35 (the C elegans Retinoblastoma protein homolog), and ubc-18 (a ubiquitin-conjugating enzyme) play partially redundant functions to control pharyngeal morphogenesis[17]. Indeed, lin-35/Rb; ubc-18 double mutants exhibit a synthetic pharyngeal phenotype; that is, failure to undergo pharyngeal primordium elongation, typically failing already at the reorientation step during which the anterior epithelial cells of the primordium should align their long axis with the dorsoventral axis of the embryo[19]. The ubc-18 and pha-1 also both show strong synthetic pharyngeal phenotypes when combined with class B synthetic multivulval (SynMuv) genes. The SynMuv genes form two molecularly heterogeneous classes (class A and B) of genes that contribute redundantly to vulva development; class B SynMuv genes obviously also play a hitherto unknown role in pharyngeal development that is redundant with both ubc-18 and pha-1[17,19].
pha-2 The pha-2 mutant worms exhibit a late defect in pharyngeal morphogenesis that results in the two pharyngeal bulbs being next to each other rather than being separated by a narrow, nucleus-free isthmus. We cloned the pha-2 gene and found that it encodes a homeobox gene most homologous to the vertebrate Hex gene[16]. Using a PHA-2::GFP translational fusion reporter in which a pha-2 genomic fragment containing 2.7 kb of pha-2 5’UTR plus the entire gene fused to GFP at its C-terminal codon, we observed expression in several pharyngeal cells: the pm5 muscle cells that form the isthmus but have their cell bodies within the posterior bulb; the pm4 cells that make up the bulk of the metacorpus; and pharyngeal epithelial cells. As this translational fusion reporter was able to rescue the mutant pheno-type, we are relatively confident that the expression profile of the reporter reflects normal PHA-2 expression. We hypothesize that PHA-2 confers an isthmus cell identity to the pm5 muscle pharyngeal cells that express it and that form the isthmus. The main characteristic of isthmus cells is that they have a long elongated shape extending into the isthmus, but that their cell bodies are embedded within the metacorpus or posterior bulb. This isthmus cell shape likely results from directional growth of the cells occurring after the comma stage (~430 min), because at this stage there is no nuclear-free zone along the length of the elongated primordium. As Sulston et al documented, it is during the 430–490 min interval, as the emerging pharynx continues its elongation, that the pharyngeal bulbs become apparent[9].
What are the genes regulated by pha-2? Experimental evidence suggests that pha-2 acts as a repressor of ceh-22 in the pm5 cells. In wild-type animals, expression of a CEH-22::GFP reporter is downregulated in the isthmus by late embryogenesis. In contrast, in pha-2 mutants the expression of the CEH-22::GFP reporter persists and even increases in the isthmus during late embryogenesis, and also post-embryonically. Because of the late effects of the pha-2 mutation, we also surmise that at some downstream level, pha-2 acts via genes that implement the differentiation program by driving the final cell shape changes, such as cytoskeletal genes. It is perhaps worth noting that located just next to the pha-2 gene is the intermediate filament 2c gene, IF-C2 (M6.1), which is expressed in pharyngeal and intestinal desmosomes and thus likely plays a role in cell-cell connections[20]. Given that intermediate filaments are important in several morphogenesis processes, including cell elongation[21,22], there is a possibility that M6.1 contributes to pharyngeal morphogenesis. Consistent with this line of reasoning, the expression of IF-C2 begins in the late embryo, when final pharyngeal morphogenesis occurs.
ceh-22 Like vertebrate cardiac and smooth muscles, the pharyngeal muscles of C elegans do not express any of the known members of the MyoD family of myogenic factors. In addition, like vertebrate cardiac muscle cells, the pharyngeal muscles exhibit an intrinsic rhythmic contraction activity that does not depend on any neuronal input. Two myosin heavy chain genes myo-1 and myo-2 are specifically expressed in pharyngeal muscles. In 1994, Okkema and Fire characterized the myo-2 promoter and identified a transcription factor that binds this promoter and regulates its expression in pharyngeal muscles[23]. This transcription factor was CEH-22, a homeobox protein most homologous to the vertebrate Nkx2.5 and the Drosophila tinman, which regulate heart development in their respective organisms. Furthermore, expression of the zebrafish nkx2.5 gene in C elegans can activate myo-2 and can rescue the ceh-22 mutant, suggesting that ceh-22 and nkx2.5 share a conserved molecular function[3]. Somewhat confusingly, the phenotype of the ceh-22 mutant includes a slightly abnormal pharyngeal shape (slight thickening of the isthmus), but no defect in the expression of myo-2, suggesting that other regulatory pathways act in parallel with ceh-22 to regulate myo-2[24]. Okkema et al showed that PHA-1 itself also directly regulates the myo-2 gene, and that a pha-1; ceh-22 double mutant is more severe than either mutant alone; the early pharyngeal 3NB12 antigen is not even expressed[24].
Development of the pharyngeal neurons
Pharyngeal nervous system overview The mature pharynx contains 20 neurons. Each establishes a unique and predictable morphology that is reproducible from worm to worm. The pharyngeal nervous system is organized into four general structures: two subventral nerve cords, one dorsal nerve cord, and one circular pharyngeal ring, which is located within the posterior half of the metacorpus and to which 12 neurons contribute processes[6]. The synapses and gap junctions made by the pharyngeal neurons have also been described and thus a basic wiring diagram of the pharyngeal network exists[6]. Speculations that the pharyngeal neural network plays an important role in regulating the pumping activity, as has been postulated for the intrinsic cardiac ganglia in the vertebrate heart[25], are stunted by the above-mentioned observation that most of the pharyngeal neurons can be laser-ablated without visible effects on pharyngeal behavior.
How does the intricate network of axon trajectories and synapses become established within the small cramped space of the developing pharynx? In particular, it is important to re-emphasize that the pharyngeal neurons, in contrast to body neurons, are not projecting between a basal lamina and an epithelial cell. Rather, they project within muscle cell folds, directly in contact with the muscle cell surface. Does this rather unique substrate for the neurons involve guidance cues different from those guiding body neurons? Also of importance is the fact that the pharyngeal neuron cells are already present in undifferentiated form within the spherical pharyngeal primordium. This offers intriguing developmental possibilities. For example, the pharyngeal neurons can take advantage of instructive interactions between cells that are neighbors within the primordium but are widely separated in the mature pharynx.
Establishing axon trajectories without growth cones We have shown that the M2 pharyngeal axons establish their trajectories via at least two independent mechanisms[26]. The straight proximal M2 trajectory (between the cell body, through the isthmus, and reaching into the metacorpus; Figure 2) does not depend on genes that act as axon guidance cues or that are important for growth cone functions. Thus, this proximal straight trajectory is established in a growth-cone-independent manner. We have suggested that the M2 cell forms, within the primordium, a physical connection with some neighboring cell that is ultimately located within the metacorpus, and that these connections elongate to form the proximal M2 axon trajectory as the primordium undergoes morphogenesis. It is a long-standing observation that mechanical tension exerted on neuronal cells can induce formation of a projection that can elongate rapidly as tension is maintained, “as a fishing line from a reel”[27,28]. Such mechanically induced axon formation and elongation can take place even when growth cone function is impaired[29]. This would be a process similar to the scenario that Sulston et al described for the pharyngeal gland cell g1 (discussed earlier), and also similar to the immature sensilla neurons that, after contacting the tip of the head, move posteriorly while laying down their dendritic processes[9]. The ability of a neuronal cell body to migrate and leave an extending axon behind has also been attributed to vertebrate facial moto-rneurons, although that particular case involves the movement of the cell nucleus (“nucleokinesis”) into a dendrite[30]. In Drosophila, the larval optic nerve undergoes a period of elongation by intercalation of membrane as the neuron cell body and a distant guidepost cell move away from each other; later, a growth-cone-dependent process completes the establishment of the distal trajectory[31]. Similarly, neurons of the larval imaginal leg disc also lengthen axons in keeping with the vast leg morphogenesis process[32].
Distal ends are established using a growth cone Axon trajectories are usually established by specialized structures at their growing ends, the growth cones, that sense the molecular environment and interpret guidance cues so as to migrate along the correct paths[33]. The distal end of the M2 axon, which exhibits a complex trajectory within the metacorpus (first turning outward laterally, then dorsally, before extending towards the midline to establish a gap junction with the contralateral M2 neuron), depends on basic growth cone function genes (unc-73, unc-51) as well as several well-known axon guidance cues and receptors for these cues (sax-3, slt-1, unc-6, unc-5, unc-40)[26]. It is therefore our conclusion that the rough trajectories of pharyngeal neurons may be established during morphogenesis in the absence of growth cones, relying instead on cell-cell contacts that are stretched to form axons during cell movements, but that fine-tuning of the ends of the trajectories depends on functional growth cones and multiple guidance cues. A screen for mutants with abnormal M2 trajectories allowed the isolation of several mnm (M neuron morphology abnormal) mutants, three of which specifically affected the formation of the distal M2 ends (Figure 2)[26]. These mutations likely affect the function of genes important for the function/guidance of the M2 growth cones.
An instructive developmental role for neurons? Interestingly some of the pharyngeal neurons themselves may act as sources of guidance cues. At least 2 of the pharyngeal neurons likely play developmental roles. The interneuron I5 is a source of UNC-6[34] that is likely to be important for the guidance of other axons, notably M2, which exhibits abnormal trajectories in a unc-6 mutant background. The interneuron I4 expresses UNC-129[35], also a secreted guidance molecule. Hence, it seems likely that many of the apparently unimportant pharyngeal neurons (most neurons of the pharynx can be ablated in the adult worm without impairing pharyngeal function) play an important role during development. It would be interesting to ablate the neuronal precursor in the embryo prior to pharyngeal morphogenesis end, and thus test directly the hypothesis that these neurons play an instructive developmental role. It would also be interesting to determine whether this is a function that neurons play in other developing organs and other organisms.
References
- Avery L, Shtonda BS. Food transport in the C. elegans pharynx. J Exp Biol 2003;206:2441-57.
- Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron 1989;3:473-85.
- Haun C, Alexander J, Stainier D, Okkema PG. Rescue of Caenorhabditis elegans pharyngeal development by a vertebrate heart specification gene. Proc Natl Acad Sci USA 1998;95:5072-5.
- Portereiko MF, Mango SE. Early morphogenesis of the Caenorhabditis elegans pharynx. Dev Biol 2001;233:482-94.
- Graham PL, Johnson JJ, Wang S, Sibley MH, Gupta MC, Kramer JM. Type IV collagen is detectable in most, but not all, basement membranes of Caenorhabditis elegans and assembles on tissues that do not express it. J Cell Biol 1997;137:1171-83.
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 1976;275:299-325.
- Avery L, Thomas JH. Feeding and defecation. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. II. New York: Cold Spring Harbor Laboratory Press; 1997. p 679–716.
- Avery L. Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J Exp Biol 1993;175:283-97.
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 1983;100:64-119.
- Avery L, Horvitz HR. A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant. Cell 1987;51:1071-8.
- Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol 2003;253:309-23.
- Leung B, Hermann GJ, Priess JR. Organogenesis of the Caenorhabditis elegans intestine. Dev Biol 1999;216:114-34.
- Kalb JM, Lau KK, Goszczynski B, Fukushige T, Moons D, Okkema PG, et al. pha-4 is Ce-fkh-1, a fork head/HNF-3α, β, γ homolog that functions in organogenesis of the C elegans pharynx. Development 1998;125:2171-80.
- Mango SE, Lambie EJ, Kimble J. The pha-4 gene is required to generate the pharyngeal primordium of Caenorhabditis elegans. Development 1994;120:3019-31.
- Gaudet J, Mango SE. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science 2002;295:821-5.
- Mörck C, Rauthan M, Wågberg F, Pilon M. pha-2 encodes the C. elegans ortholog of the homeodomain protein HEX and is required for the formation of the pharyngeal isthmus. Dev Biol 2004;272:403-18.
- Fay DS, Qiu X, Large E, Smith CP, Mango S, Johanson BL. The coordinate regulation of pharyngeal development in C. elegans by lin-35/Rb, pha-1, and ubc-18. Dev Biol 2004;271:11-25.
- Granato M, Schnabel H, Schnabel R. Genesis of an organ: molecular analysis of the pha-1 gene. Development 1994;120:3005-17.
- Fay DS, Large E, Han M, Darland M. lin-35/Rb and ubc-18, an E2 ubiquitin-conjugating enzyme, function redundantly to control pharyngeal morphogenesis in C. elegans. Development 2003;130:3319-30.
- Karabinos A, Schulze E, Klisch T, Wang J, Weber K. Expression profiles of the essential intermediate filament (IF) protein A2 and the IF protein C2 in the nematode Caenorhabditis elegans. Mech Dev 2002;117:311-4.
- Ding M, Woo WM, Chisholm AD. The cytoskeleton and epidermal morphogenesis in C. elegans. Exp Cell Res 2004;301:84-90.
- Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol 2004;6:699-706.
- Okkema PG, Fire A. The Caenorhabditis elegans NK-2 class homeodomain CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development 1994;120:2175-86.
- Okkema PG, Ha E, Haun C, Chen W, Fire A. The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development 1997;124:3965-73.
- Randall DC. Towards an understanding of the function of the intrinsic cardiac ganglia. J Physiol 2000;528:406.
- Mörck C, Axäng C, Pilon M. A genetic analysis of axon guidance in the C. elegans pharynx. Dev Biol 2003;260:158-75.
- Bray D. Axonal outgrowth in response to experimentally applied tension. Dev Biol 1984;102:379-89.
- Zheng J, Lamoureux P, Santiago V, Dennerll T, Buxbaum RE, Heidemann SR. Tensile regulation of axonal elongation and initiation. J Neurosci 1991;11:1117-25.
- Lamoureux P, Altun-Gultekin ZF, Lin C, Wagner JA, Heidemann SR. Rac is required for growth cone function but not neurite assembly. J Cell Science 1997;110:635-41.
- Lambert de Rouvroit C, Goffinet AM. Neuronal migration. Mech Dev 2001;105:47-56.
- Schmucker D, Jäckle H, Gaul U. Genetic analysis of the larval optic nerve projection in Drosophila. Development 1997;124:937-48.
- Jan YN, Ghysen A, Christoph I, Barbel S, Jan LY. Formation of neuronal pathways in the imaginal discs of Drosophila melano-gaster. J Neurosci 1985;5:2453-64.
- Dickson BJ. Molecular mechanisms of axon guidance. Science 2002;298:1959-64.
- Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 1996;16:35-46.
- Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG. Pioneer axon guidance by UNC-129, a C. elegans TGF-β. Science 1998;281:706-9.
