Swietenia mahagony extract shows agonistic activity to PPARγ and gives ameliorative effects on diabetic db/db mice
Introduction
Peroxisome proliferator-activated receptor (PPAR) is a ligand-binding transcriptional regulatory factor, which belongs to the nuclear receptor superfamily and regulates the expression of a group of genes involving glucose and lipid metabolism. There are three PPAR subtypes, commonly designated as PPARα, PPARβ (δ), and PPARγ[1,2]. The functions of these PPAR isoforms, after activation by drugs (anti-inflammatory agents, fibric acids) and fatty acid derivatives (including prostaglandins and plasticizers), include an increase in lipid and cholesterol metabolism, adipocyte differentiation, and an improvement in insulin sensitivity[1,3,4]. PPARγ is the most extensively studied PPAR subtype. It has been demonstrated that PPARγ is the receptor of the thiazolidinedione (TZD) class ligands[5]. Among the TZD type antidiabetic drugs, rosiglitazone and troglitazone are potent adipocyte-differentiating agents, which activate ap2 gene expression in a PPARγ-dependent manner[6]. As PPARγ ligands may regulate the adipogenesis, they can be designed and modified for the treatment of cardiovascular disease and diabetes mellitus[1,7], and PPARγ has been an attractive target for new drug discovery. To date, several types of PPARγ agonist with new structures have been developed, as though few can be clinically used[8]. In fact, the search for new PPARγ agonists has long been an alluring project.
Nature remains as a source for organic structures with unparalleled diversity, and the enormous importance of natural product is obvious. In recent years, there has been growing interest in alternative therapies and the therapeutic use of natural products, especially those derived from plants. In fact, natural products, containing inherently more structural diversity than synthetic compounds, have been the major resources of bioactive agents and will continue to play an important role in the discovery of new drugs[9]. Encouraged by these facts, we have recently focused on finding of PPARγ agonist on the basis of natural resource exploration. This present study was to test the PPARγ agonistic activity of SmE and observe the ameliorative effects of Smietenia mahagony extract (SmE) on diabetic db/db mice.
Materials and methods
Materials The plant material was purchased from Indonesia and the specimen (N
Animals The db/db mice were supplied by Shanghai BK Corporation. The male mice, 40–50 g, were sanitary, and allowed free access to water during the experiment.
Extract of Swietenia mahagony Powdered S mahagony seeds (0.8 kg) was refluxed with EtOH (95%, 2 L) for 2 h three times, then filtered. The combined filtrate was concentrated under reduced pressure and partitioned by EtOAc to obtain EtOAc fraction (40 g).
PPARγ agonists assay Yeast liquid synthetic dropout medium without leucine and tryptophan (T-L-) was prepared referring to Yeast Protocol Handbook[10]. SmE (50 µg/L) and rosiglitazone (0.1 µmol/L) were dissolved in Me2SO for assay use.
The yeast two-hybrid system was established for identifying PPARγ agonist by our laboratory[11]. The yeast strain AH109 named p1c2, harboring the expression plasmid pGADT7-CBP and PGBKT7-PPARγLBD, was used for PPARγ agonist screening [12].
Yeast clone p1c2 was inoculated into 2 mL T-L-liquid medium from a plate, then incubated at 30 ºC overnight (16–18 h) with shaking (250 r/min). After vortexing and recording OD600, the cell culture was diluted with T-L-liquid medium until its OD600 reached about 0.05. Subsequently 5 µL of Me2SO or drug was added to 495 µL of diluted yeast culture, test cultures were incubated at 30 °C overnight (14–16 h), and then α-Galactosidase Assay was performed[10].
Blood glucose level measurement The basal blood glucose levels of 40 male db/db diabetic mice were measured everyday for 7 d, and 24 mice showing comparatively steady blood glucose level were screened out. These mice were divided into three groups (n=8) according to the blood glucose level. The mice were given 0.5% CMC-Na (10 mL/kg) in the control group, SmE (1000 mg/kg) or rosiglitazone (10 mg/kg) in the treated group by ig everyday for 2 weeks. The blood glucose concentrations were measured using a blood glucose level monitor (OneTouchR Ultra, Shanghai) every other day. Data were analyzed using the NDST8.8W analysis program.
Statistical analysis The results were expressed as mean±SD and analyzed by unpaired t-test.
Results
PPARγ ligand-binding activity of SmE SmE exhibited moderate agonistic activity to PPARγ, and its relative α- galactosidase intensity from the yeast two-hybrid assay was 2.13 at the concentration of 50 µg/L, which was approximately half that of rosiglitazone 35.7 µg/L (0.1 µmol/L), a potent synthetic PPARγ agonist (Figure 1), whose relative α-galactosidase intensity is around 4.29.
Effect of SmE on diabetic db/db mice After 14 d of feeding, the blood glucose levels decreased markedly in the both SmE- and rosiglitazone-treated groups compared with the control group. The blood glucose concentration of db/db mice in the control group was 17.03 mmol/L, yet in the treated groups 7.58 mmol/L and 6.98 mmol/L respectively (Table 1). In comparison with the control group, the blood glucose levels decreased 55.49% (P<0.01) in the SmE-treated group and 59% (P<0.01) in the rosiglitazone-treated group.
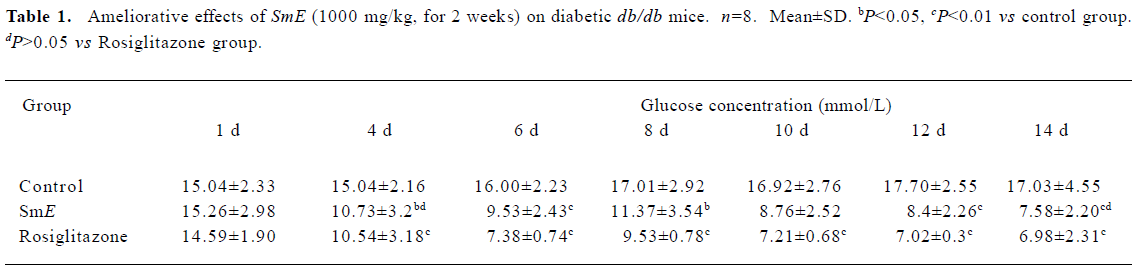
Full table
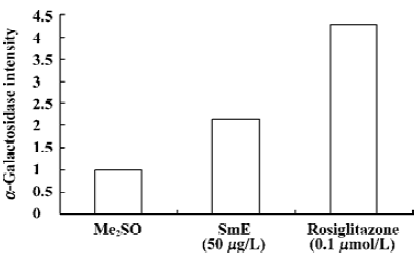
Discussion
Swietenia mahagony is a large, medicinally and economically important timber tree native to the West Indies. The seeds of this plant are used for the treatment of hypertension and malaria as a folk medicine in Indonesia[13]. In the present study, we tested the effect of S mahagony on the PPARγ agonistic activity and the amelioration of the blood glucose level in type-II diabetic mice, a representative insulin resistance syndrome. With the help of the yeast two-hybrid system assay, the possible anti-diabetic mechanism of Swietenia mahagony was proposed. The results in vivo showed that Sm E exhibited moderate effects on decreasing the blood glucose levels of the diabetic db/db mice. These results may give us a new hint that SmE might be used as a potential agent for diabetic therapy with its PPARγ transcriptional regulatory function as one of the in vivo mechanisms even though there may be existed other efficient components in SmE against other targets for diabetic therapy.
Acknowledgement
Project supported by Kuancheng Wang Fundation of Chinese Academy of Sciences (2003), Shanghai Basic Research Project from the Shanghai Science and Technology Commission (N
References
- Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem 2000;43:527-50.
- Kerstern S, Desverge B, Wahli W. Roles of PPARs in health and disease. Nature 2000;405:421-4.
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutation Res 2000;448:121-38.
- Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet 1999;354:141-8.
- Reginato MJ, Lazar MA. Mechanisms by which thiazolidinediones enhance insulin action. Trends Endoc Metab 1999;10:9-13.
- Hulin B, McCarthy PA, Gibbs EM. The glitazone family of antidiabetic agents. Curr Pharm Des 1996;2:85-102.
- Barry GS, William JH. Recent advances in peroxisome proliferator-activated receptor science. Curr Med Chem 2003;10:267-80.
- Murphy GJ, Holder JC. PPARγ agonists: therapeutic role in diabetes, inflammation and cancer. Trends Pharm Sci 2000;21:469-74.
- Shen JH, Xu XY, Cheng F, Liu H, Luo XM, Shen JK, et al. Virtual screening on natural products for discovering active compounds and target information. Curr Med Chem 2003;10:2327-42.
- Yeast protocols handbook. Available from: http://www.bdbiosciences.com/clontech/techinfo/manuals/PDF/PT3024-1.pdf.
- Chen Q, Chen J, Sun T, Shen JH, Shen X, Jiang HL. A yeast two-hybrid technology based system for the discovery of PPARγ agonist and antagonist. Anal Biochem 2004;335:253-9.
- Taniguchi T, Mizukami J, inventors. Method for identifying or screening agonist and antagonist to PPAR. US patent 6 365 361. 2002 Aug 23.
- Kadota S, Marpaaung L, Kikuchi T, Ekimoto H. Constituents of the seeds of Swietenia mahagoni JACQ. III. Structures of mahonin and secomahoganin. Chem Pharm Bull 1990;38:1495-500.
