Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action
Introduction
Ginseng, the root and rhizome of Panax ginseng C A Meyer, has been used as a tonic remedy in Chinese traditional medicine for over 2000 years. The word panax is derived from panacea, which means cure-all and longevity. In 200 AD when this herb was described in the oldest Chinese materia medica book: Shen Nong Ben Cao Jing, the author depicted many pharmacological functions of ginseng, including both nootropic and anti-aging effects. Since then, ginseng has been widely used as a supplemental medicine. However, it is neither a tonic nor a remedy for a certain disease.
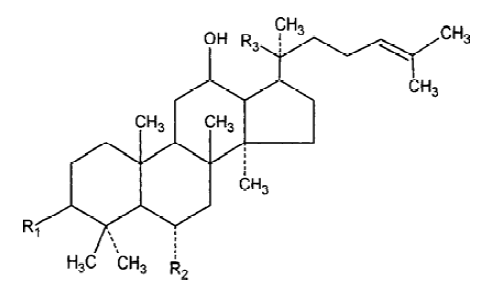
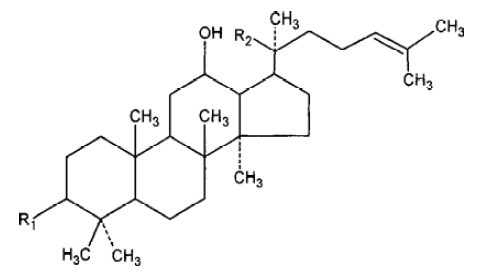
With the development of modern technology, more and more active principles have been isolated and purified from this herb, and the field of ginseng research has drawn intense attention in Asia, Europe, and the United States. Every year, there are hundreds of papers published and new uses for ginseng discoveried. More than 40 ginsenosides have been isolated from several species of ginseng. Among all the active ingredients isolated from ginseng, we selected Rg1 and Rb1 for study because Rg1 and Rb1 are the representative constituents of panax ginseng and American ginseng, respectively. Rg1 and Rb1 have different chemical structure: Rg1 is panaxtriol with 2 sugars, while Rb1 is panaxtriol with 4 sugars. The chemical structure of Rg1 and Rb1 are shown in Figure 1 and 2. Many researchers believe that they share many beneficial effects of ginseng, including alleviating learning and memory impairment, reversing pathological and physiological changes induced by stress and aging, etc. In the review, we take an overview of the proven anti-amnestic and anti-aging effects of both compounds and discuss their possible mechanisms in detail.
Anti-amnestic effects
Various memory-impairment models have been used to evaluate how ginseng and its active ingredients affect learning and memory. In a passive avoidance test, ginsenoside Rg1, (25–100 mg/kg) improved learning and memory’s acquisition, consolidation and retrieval, which were impaired by anisodine, cycloheximide, and alcohol respectively, indicating that Rg1 can improve all stages of memory. To study the effect of Rg1 on the learning and memory induced by β-amyloid (25–35), passive avoidance and performance in Morris water maze were assayed after final treatment, and we found that Rg1 at 5–10 mg/kg significantly decreased latency and swimming distance, and improved corresponding changes in search strategies in the Morris water maze and increased step-through latency also. Rg1 significantly improved memory deficits in aged rats, ovariectomizde rats, and cerebral ischemia-reperfesion rats. Results of the present study showed that ginseng extract and ginsenoside Rg1, Rb1 (25–100 mg/kg) facilitated acquisition and retrieval of memory. Moreover, they also antagonized memory loss and cognitive deficit under various pathological conditions such as cerebral ischemia and dementia. However, the nootropic effect of Rg1 was generally stronger than that of Rb1. All the beneficial effects of ginsenosides observed in the memory-impairment models have been repeated many times in different labs[1–7].
Long-term potentiation (LTP) was first observed in 1973. Since then, it has been thoroughly investigated. Many scientists believe that LTP in the hippocampus is the key form of long-lasting synaptic plasticity and it connects the behavior of learning and memory with the plasticity of neurons. Therefore, LTP has been considered as an important index of cognitive activities in cellular and synaptic levels. In Zhang’s lab[8,9], both total ginsenosides (25–100 mg/kg, ip) and ginsenoside Rg1 (10 and 100 nmol/L, icv) were found to improve the basic synaptic transmission and increased the amplitude of LTP induced by high-frequency-stimulation (HFS) in anesthetized rats. In contrast, although ginsenoside Rb1 (10 and 100 nmol/L, icv) administered 30 min after tetanic stimulation was found to increase population spike (PS) amplitude[10], it exerted an inhibitory effect on LTP induced by HFS when given at the same time of stimulation and showed no influence on basic synaptic transmission. Further research suggests that nitric oxide (NO) synthesized by nitric oxide synthase (NOS), especially by nNOS, was involved in synaptic transmission improving the effect of Rg1[11]. In order to eliminate the influence of anesthetics on synaptic transmission, rats were chronically implanted with a stimulation electrode in the perforated path and a recording electrode in the granule cell of dentate gyrus. After Rg1 was administrated (10 and 30 mg/kg, ip) for 12 d, an extracellular recording technique was used to record PS. The results demonstrated that Rg1 significantly increased the sensitivity of evoking PS and the amplitude of PS in freely moving rats[11].
Mechanisms underlying the nootropic effect of ginseng First, ginsenoside might potentiate the cholinergic system in the central nervous system (CNS). Acetylcholine (ACh) is a very important neurotransmitter in the brain and its scarcity often leads to learning and memory impairment. In Zhang’s lab, Rg1 and Rb1 were found to enhance cholinergic system’s function through two different pathways: first, both ginsenosides showed the ability to increase the density of central M-cholinergic receptors, although they showed no specific binding to the receptors. Second, Rg1 and Rb1 increased the level of ACh in the CNS, which might be the result of ginsenoside-induced enhancement on choline acetyltransferase activity and inhibition on acetylcholine esterase activity[2,12]. Meanwhile, Banishin et al reported that both ginsenosides facilitated cholinergic neurotransmission by increasing cholinergic metabolism in the CNS[13].
It is very interesting to point out that according to existing theory, receptor density is directly dependent on transmitter activity in the brain. For instance, muscarinic receptor level falls in response to the administration of its agonists, while there is a rise in response to chronic administration of muscarinic antagonists. However, findings in Zhang’s lab show simultaneous increases in M receptor density and Ach level, which is not in line with previous observations[14]. So, maybe a new theory should be provided to elucidate the effects of ginseng on the cholinergic system.
First, both Rg1 and Rb1 were found to increase protein biosynthesis in the mouse brain[12]. This finding could also explain ginseng’s function on memory consolidation. In addition, the increment of M-receptor density could be one of the secondary effects of the enhancement of protein synthesis.
Second, ginsenoside may interfere with immediate early genes. The c-fos proto-oncogene is the prototype of the early-response class of genes and its expression can be considered as a marker of neuronal activity. Rg1 was found to significantly increase the expression of c-fos gene in both young and old rats[15]. Furthermore, it had the ability to increase cyclic adenosine monophosphophate (cAMP) level in rat hippocampus. This might provide another explanation for the nootropic effect of Rg1.
Third, apoptosis or programmed cell death is a process by which a cell actively commits suicide under tightly controlled circumstances. Apoptosis is required for maintenance of the homeostasis and is essential in physiological processes such as development, differentiation and aging. However, defective regulation of apoptosis might play a role in the etiology of cancer, acquired immunodeficiency syndrome, autoimmune diseases and neural degenerative disorders. Therefore, pharmacological manipulation of this process may offer new possibilities for the prevention and treatment of many illnesses. In 1997, Zhang’s lab reported firstly that Rg1 at concentration of 1 µmol/L and 10 µmol/L inhibited apoptosis induced by withdrawing serum from the culture system of primary cortical neurons[16]. In vivo study, an anti-apoptotic effect of Rg1 was seen in aged rats (24–27 month). Further study showed that mechanisms underlying Rg1’s effect on apoptosis involved decreasing NO content and NOS activity, reducing intracellular calcium concentration and enhancing superoxide dismutase (SOD) activity. Researchers from the same lab also found that both NOS expression and the activity of NOS enhanced significantly in aged rats (27 months old), which leads to the increment of NO concentration in rat’s cortex. This change was reversed by Rg1 administration (20 and 40 mg/kg daily for 5 d)[17]. Taken together with previous in vitro results, they confirmed that NO played a role in the acceleration of senescence and that the inhibitory effect of Rg1 on NOS activity might provide an explanation for its anti-aging function.
Recently, the anti-apoptotic effect of Rg1 on neurons was further proved by both in vivo and in vitro experiments done in other labs[18,19]. The authors of these studies suggested that this effect of Rg1 might attribute to enhancing the ratio of Bcl-2 to Bax protein and inhibiting activation of caspase-3.
Fourth, ginsenoside may modulate synaptic plasticity. Synapses are the essential structure in the CNS through which signals are transmitted, processed and integrated among neurons. The plasticity of synaptic function is also regarded as one of the most important mechanisms underlying the process of learning and memory. Interestingly, in weaning mice administered with Rg1 and Rb1 for 14 d, the thickness of cortex and density of synapses in the hippocampal CA3 region were significantly increased (Table 1)[20]. Meanwhile, Rg1 was found to induce the sprouting of mossy fiber and the expression of the growth-associate protein (GAP-43) dose-dependently in hippocampus in adult rats (Figure 3)[9]. Furthermore, both Rg1 and Rb1 were found to increase hippocampal synaptic density[21]. These experiment proofs provided morphological and physiological evidence to support nootropic effect of ginseng.
Finally, ginsenoside, especially Rg1 might regulate the proliferation and/or surviving ability of neural progenitor cells. A neural progenitor cell (NPC) or Neural stem cell (NSC) is a special kind of neural cell. Previous studies have shown that NPC existed in certain areas in the adult brain as well as in developing brain[22]. Recently, several rodent studies have suggested that the proliferation ability of NPC was reduced in association with age or diseases related cognitive decline[23–25]. Hence, many scientists hypothesize that damage of neurogenesis may be one of the elementary reasons for function-declines in aging and neurodegenerative diseases. Recently, Shen et al[26] reported that Rg1 enhanced proliferation ability of rodent hippocampal progenitor cells both in vitro and in vivo. Incubation of neural progenitor cells with Rg1 resulted in significant increase in absorbency value, [3H]thymidine incorporation and the number of proliferating progenitor cell spheres. In addition, Rg1 administrated for 2 weeks (ip) led to marked enhancement of the number of dividing cells in hippocampus of adult mice. Meanwhile, the same authors found that Rg1 systemically administered not only promoted cell proliferation, but also enhanced the surviving rate of newborn cells in the dentate gyrus of adult gerbils suffered from transient global ischemia (Figure 4)[27]. The authors suggested that influencing the NPC function might serve as one of the elementary mechanisms underlying pharmacological effects of Rg1 and ginseng on the CNS.
To summarize, the nootropic effect of ginseng and ginsenosides has been proved in both behavioral and electrophysiological studies, which is unique among existing nootropic drugs.
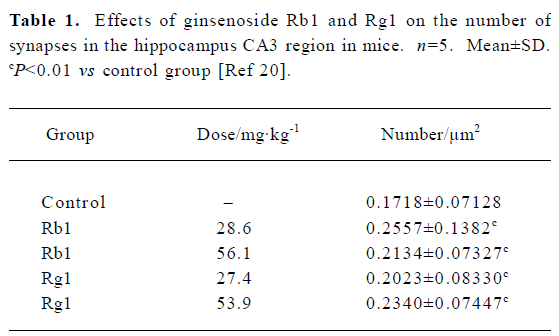
Full table
Anti-aging effects
Anti-stress effects Various kinds of stress, especially chronic stress, cause many diseases and accelerate aging, so anti-stress drugs are in high demand. Ginseng has long been considered to act as an adaptogen, but the mechanisms underlying its effect are still unclear. Recently, acute, chronic, and repeated stress models were used to observe the effects of ginsenosides on stress.
Repeated hanging stress was used to observe the effect of stress on the reproduction-endocrine system. The results indicated that repeated stress reduced sexual behavior (licking, mounting, and mating), decreased plasma androgen or estrogen levels and increased corticorsterone level in male and female mice. Treatment with ginsenoside Rb1 at dosage of 2.5, 5, and 10 mg/kg prior to stress prevented the decrease of sexual behavior and the increase of corticorsterone. Rb1 brought the concentration of plasma sexual hormones back to normal level [28]. Further study proved that Rb1 (2.5, 5 and 10 mg/kg, ip for 14 d) significantly improved sexual function in mice. The mechanism underlying its “zhuang yang” effects might be the activation of NO/cyclic guanosine monophosphophate (cGMP) pathway in mice corpus cavernosum[29].
Dysfunction of reproductive-endocrine system is often accompanied by the degeneration of cognitive processes in middle and late age. Therefore, the influence of chronic stress on cognitive abilities in mice was observed. The result show-ed that 60 consecutive days of stress caused a neuronal damage as well as cognitive impairment. Further study suggested that chronic stress decreased brain-derived neurotrophic factor and neurotrophin-3 (NT-3) protein expression throughout the brain area especially in the hippocampus. In addition, stress also induced the release of noradrenaline in the homogenates of the hippocampus and the hypo-thalamus. Similarly, ginsenoside Rb1 showed the ability to reverse all the pathological changes induced by stress[30]. However, ginsenoside Rg1 had no effect on both repeated and chronic stress-related changes. Instead, it accelerated stress-induced pathological changes in mice, showing another difference between Rg1 and Rb1. Based on these results, Rb1 is believed to be the main anti-stress principle in ginseng and it might be a promising candidate for prevention and treatment of stress-related diseases.
Immunoregulatory effects It has been well documented that aging leads to a substantial decline of T cell function. The possible reasons for the decline include the inability of lymphocytes to proliferation in response to mitogenic stimulation and the decrease of interleukin-2 (IL-2) production. Rg1 given at the concentration of 20 mg/kg in vivo and 10 µmol/L in vitro enhanced the proliferation of lymphocytes and the production of IL-2 in aged rats[31]. However, it had no influence on the immune function in young and adult rats. Thus, it is reasonable to consider Rg1 as an “immuno-regulator” rather than an “immunopotentiating agent”. Further investigation suggested that the mechanism underlying Rg1’s effect on immune function in aged rats might be involved in increasing cAMP and cGMP levels in lymphocytes[32].
In contrast, Rb1 had no effect on the decline of immune function in aged rats, which was the third difference found between Rg1 and Rb1.
Other effects In the previous paragraphs, we summarized the pharmacological effects of ginseng, especially ginsenoside Rg1 and Rb1, and the possible mechanisms of these actions. Recent studies have shown that ginseng saponins are a diverse group of steroidal saponins that demonstrates the ability to target a myriad of tissues and produce an array of pharmacological responses, such as anti-osteoporosis, anti-platelet aggregation, anti-arrhythmia, and anti-cancer[7,33–39]. Furthermore, besides ginsenosides, there are also other constituents existing in ginseng, including glucoside, protein, poly-peptides, amino acids, vitamins, etc. Their pharmacological functions have been investigated too[40,41].
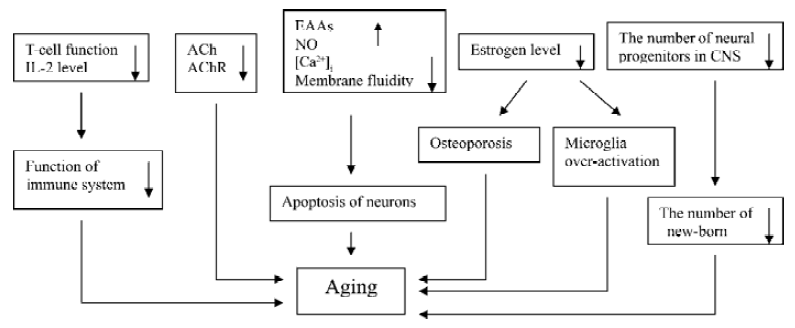
Senescence is a physiological process and cannot be reversed. Many factors act together in this process (Figure 5). As discussed above, ginseng, especially ginsenoside Rg1 and Rb1, have the ability to interfere almost all of the pathways that accelerate aging process. Besides the effects we mentioned above, some researchers also found that both ginsenosides to some extent inhibited lipid peroxidation induced by VitC-reduced nicotinamide adenine dinucleotide phosphate and Fe2+-cysteine in rat liver and brain micro-some; reduced the concentration of intercelluar calcium; and prevented hippocampal neurons from the damage induced by excitatory amino acids[42–46]. All those findings may prove the anti-aging and nootropic effects of this ancient herb.
Concluding remarks
Rg1 and Rb1 are believed to be the main active principles in ginseng. As we discussed above, both Rg1 and Rb1 showed similar effects in improving learning and memory, increasing Bmax of M-cholinergic receptors, and accelerating cerebral protein and ACh biosynthesis. However, they do behave differently in some areas. For example, Rg1, but not Rb1, enhanced basic synaptic transmission and magnitude of LTP induced by HFS, and showed immunoregulatory action in aged rats as well as an anti-osteoporosis effect in ovariectomized rats. However, Rb1 showed anti-stress effect in acute, chronic, and repeated stress models, whereas Rg1 aggravated stress-induced damage; in addition, Rb1 protected mice against low temperature damage, and Rg1 showed no such effect at the same dose. For the reason of the function differences between the two ginsenosides, some researchers hypothesize it may be due to the difference between their chemical structures (Rb1 has two more glucose as compared to Rg1).
With the application of modern scientific technology, the research of ginseng has made big progress. Ginseng is believed to be the most unique traditional medical herb because it contains the maximum numbers of active constitu-ents, has the most extensive pharmacological effects and specific mechanism of actions in Chinese materia medica.
Chemists have already acquired nearly 40 kinds of ginsenosides from various parts of this plant and new structures continue to identified[47–52]. Becuase ginsenosides and other constituents of ginseng produce different effects and a single ginsenoside initiates multiple actions in the same tissue, the overall pharmacology of ginseng is complicated. Therefore, we must investigate the effects of individual saponin and its mechanisms of actions one by one. But we still cannot synthesize any ginsenosides because of the complexity of their structures. Currently, the only way to prepare active principles from ginseng is to separate and purify them from various parts of the plant. But with the application of modern technology, the yield of some ginsenosides such as Rb1, Rg1, Rg3, and Re has reached up to 1 kg in a number of Chinese corporations. Hence, we can predict that the preparation of active constituents in ginseng has reached the level of industrial production, which makes it possible to further investigate this mysterious herb.
Ginseng and ginsenoside can increase immune function, enhance central cholinergic system function, inhibit free radical and NO generation, and promote proliferation of rodent progenitor cells in vitro and in vivo (Figure 5). These effects benefit aged people and aging-ralated diseases.
References
- Chang SY, Kuang PG, Zhang JT, Chen YY, Yu CM. Memory facilitation induced by gynostemma, pentaphyllum and gypensode III (ginsenoside b1) in mice. Chin Pharmacol Bull 1988;4:358-61.
- Zhang JT, Qu ZW, Liu Y, Deng HL. Preliminary study on antiamnestic mechanism of ginsenoside Rg1 and Rb1. Chin Med J 1990;103:932-8.
- Qiu Y, Du GH, Qu ZW, Zhang JT. Protective effects of ginsenoside on the learning and memory impairment induced by transient cerebral ischemia-reperfusion in mice. Chin Pharmacol Bull 1995;11:299-302.
- Zhang DS, Zhang JT. Effects of total ginsenoside on learning and memory impairment induced by beta-amyloid peptide (25-35). Chin Pharmacol Bull 2000;16:422-5.
- Wang XY, Zhang JT. Effects of ginenoside Rg1 on learning and memory impairment induced by β-amyloid peptide (25-35) and its mechanism of action. Acta Pharm Sin 2001;36:1-4.
- Mook-Jung I, Hong HS, Boo JH, Lee KH, Yun SH, Cheong MY, et al. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res 2001;63:509-15.
- Chen J, Gong YS, Zhang JT. Effects of 17 estradiol and total ginsenoside on the spatial learning and memory impairment of ovariectomy rats. Chin Pharm J 2001;36:522-6.
- Zhang DS, Zhang JT. Effect of total ginsenoside on synaptic transmission in dentate gyrus in rats. Acta Pharm Sin 2000;35:185-8.
- Wang XY, Zhang JT. NO mediates ginsenoside Rg1-induced long-term potentiation in anesthetized rats. Acta Pharmacol Sin 2001;22:657-62.
- Wang XY, Zhang JT. Effect of ginsenoside Rb1 on long-term potentiation in the dentate gyrus of anaesthetized rats. J Asian Nat Prod Res 2003;5:1-4.
- Wang XY, Zhang JT. Effects of ginsenoside Rg1 on synaptic plasticity of freely moving rats and its mechanism of action. Acta Pharmacol Sin 2001;22:1099-102.
- Zhang JT, Liu Y, Qu ZhW, Zhang XL, Xiao HL. Influence of ginsenoside Rb1 and Rg1 on some central neurotransmitter receptors and protein biosynthesis in mouse brain. Acta Pharm Sin 1988;23:12-6.
- Banishin CG, Liu HJ, Wang LCH, Pang PKT. Ginsenosides Rb1 and Rg1 increase central nervous system cholinergic metabolism. In: Shojishibata editors. Recent advances in ginseng studies. Tokyo: Hirokawa Publishing Company 1989; 139–43.
- Burgen ASV. Regulation of acetylcholine receptors. In: Yoshida H, , eds. Advances in pharmacology and therapeutics: II. Neurotransmitters receptors. Tokyo: Pergamon press 1981; 51–2.
- Liu M, Zhang JT. Influence of ginsenoside Rb1 and Rg1 on some central neurotransmitter receptors and protein biosynthesis in mouse brain. Acta Pharmacol Sin 1996;17:171-4.
- Li JQ, Zhang XG, Zhang JT. Inhibition of apoptosis by ginsenoside Rg1 in cultured cortical neurons. Acta Pharm Sin 1997;32:406-10.
- Li JQ, Li ZK, Duan H, Zhang JT. Effect of age and ginsenoside Rg1 on nitric oxide content and nitric oxide synthase activity of cerebral cortex on rats. Acta Pharm Sin 1997;32:251-4.
- Chen XC, Chen Y, Zhu YG, Fang F, Chen LM. Protecive effect of ginsenoside Rg1 against MPTP-induced apoptosis in mouse substantia nigra neurons. Acta Pharmacol Sin 2002;23:829-34.
- Chen XC, Zhu YG, Wang XZ, Zhu LA, Huang C. Protective effect of ginsenoside Rg1 on dopamine-induced apoptosis in PC12 cells. Acta Pharmacol Sin 2001;22:673-8.
- Yang Y, Zhang JT, Shi CZ, Qu ZW, Liu Y. Study on the nootropic mechanism of ginsenoside Rb1 and Rg1 influence on mouse brain development. Acta Pharm Sin 1994;29:241-5.
- Mook-Jung I, Hong HS, Boo JH, Lee KH, Yun SH, Cheong MY, et al. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res 2001;63:509-15.
- Gage FH. Mammalian neural stem cells. Science 2000;287:1433-8.
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 1996;16:2027-33.
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001;410:372-6.
- Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Med 2002;1:125-35.
- Shen LH, Zhang JT. Ginsenoside Rg1 promotes proliferation of hippocampal progenitor cells. Neurol Res 2004;26:422-8.
- Shen LH, Zhang JT. Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of adult gerbils. Neurosci Lett 2003;344:1-4.
- Lian XY, Zhang JT. Effect of ginsenoside Rb1 on repeated stress-induced sexual deficiencies in male mice. Acta Pharm Sin 1998;33:184-7.
- Wang XY, Zhang JT. Effect of ginsenoside Rb1 on mouse sexual function and its mechanism. Acta Pharm Sin 2002;35:492-5.
- Lian XY, Zhang JT. Chronic stress and gonadectomy decrease the levels of neurotrophin-3 and brain-derived neurotrophic factor in adult mouse brain. Chin J Pharmacol Toxicol 2001;15:245-50.
- Liu M, Zhang JT. The immunoregulatory effects of ginsenoside Rg1 in aged rats. Acta Pharm Sin 1995;30:818-23.
- Liu M, Zhang JT. Studies on the mechanisms of immunoregulatory effects of ginsenoside Rg1 in aged rats. Acta Pharm Sin 1996;31:95-100.
- Chan RY, Chen WF, Dong A, Guo D, Wong MS. Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng. J Clin Endocrinol Metab 2002;87:3691-5.
- Teng CM, Kuo SC, Ko FN, Lee JC, Lee LG, Chen SC, et al. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim Biophys Acta 1989;990:315-20.
- Kimura Y, Okuda H, Arichi S. Effects of various ginseng saponins on 5-hydroxytryptamine release and aggregation in human platelets. J Pharm Pharmacol 1988;40:838-43.
- Wu W, Zhang XM, Liu PM, Li JM, Wang JF. Effects of Panax notoginseng saponin Rg1 on cardiac electrophysiological properties and ventricular fibrillation threshold in dogs. Acta Pharmacol Sin 1995;16:459-63.
- Park KH, Shin HJ, Song YB, Hyun HC, Cho HJ, Ham HS, et al. Possible role of ginsenoside Rb1 on regulation of rat liver triglycerides. Biol Pharm Bull 2002;25:457-60.
- Ota T, Maeda M, Odashima S, Ninomiya-Tsuji J, Tatsuka M. G1 phase-specific suppression of the Cdk2 activity by ginsenoside Rh2 in cultured murine cells. Life Sci 1997;60:PL39-44.
- Han R. Highlight on the studies of anticancer drugs derived from plants in China. Stem Cells 2002;12:53-63.
- Zhang JT. The retrospection and prospect of ginseng research. Acta Pharm Sin 1995;30:321-5.
- Dou DQ, Jin L, Chen YJ. Advances and prospects of the study on chmical constituents and pharmacological activities of Panax ginseng. J Shenyang Pharm Univ 1999;16:151-6.
- Huang YS, Liu Y, Qu ZW, Zhang JT. Effect of ginsenoside Rb1 and Rg1 on lipid peroxidation of rat in vitro. Acta Acad Med Sin 1989;11:460-2.
- Deng HL, Zhang JT. Anti-lipid peroxydative effect of ginsenoside Rb1 and Rg1. Chin Med J 1991;104:395-8.
- Liu M, Zhang JT. Effects of ginsenoside Rb1 and Rg1 on synaptosomal free calcium level, ATPase and calmudulin in rat hippocampus. Chin Med J 1995;108:544-7.
- Jiang XY, Zhang JT, Shi CZ. Mechanism of action of ginsenoside Rb1 in decreasing intracellular Ca2+. Acta Pharm Sin 1996;31:321-6.
- Liu M, Zhang JT. Protective effects of ginsenoside Rb1 and Rg1 in cultured hippocampal neurons. Acta Pharm Sin 1995;30:674-8.
- Wang JH, Li W, Sha Y, Tezuka Y, Kadota S, Li X. Triterpenoid saponins from leaves and stems of Panax quinquefolium L. J Asian Nat Prod Res 2001;3:293-7.
- Yang XW, Li LY, Tian JM, Zhang ZW, Ye JM, Gu WF. Ginsenoside-Rg6, a novel triterpenoid saponin from the stem-leaves of Panax ginseng CAMey. Chin Chem Lett 2000;11:909-12.
- Le TO, Quan LT, Adnyana IK. Triterpene saponins from Vietnamese Ginseng (Panax vietnamensis) and their hepatocytoprotective activities. J Nat Prod 2001;64:456-61.
- Dou DQ, Chen YJ, Liang LH, Pang FG, Shimizu N, Takeda T. Six new dammarane-type triterpene saponins from the leaves of Panax ginseng. Chem Pharm Bull 2001;49:442-6.
- Park JD, Lee YH, Kim SI. Ginsenoside Rf2, a new dammarane glycoside from Korean red ginseng (Panax ginseng). Arch Pharm Res 1998;21:615-7.
- Qiu F, Ma ZZ, Pei YP, Xu SX, Yao XS, Chen YT, et al. A new dammarane glycoside from the flower-buds of Panax ginseng CA Meyer. Chin Chem Lett 1998;9:643-5.