Wuweizisu C from Schisandra chinensis decreases membrane potential in C6 glioma cells1
Introduction
Schisandra chinensis Baill grows wild in Russia, China, Korea, and Japan. In Oriental countries, ripe Schisandra fruits have been used as a tonic[1]. The fruit of Schisandra chinensis has been found to be effective in viral and chemically-induced hepatitis[2]. It is enriched in lignans, and more than 30 lignans have been isolated from this fruit[3,4]. Several lignans, including wuweizisu C and gomisin A, have been reported to protect the liver from hepatotoxic chemicals[5–7]. Pharmacological studies of lignans have also revealed anti-inflammatory[8] and anti-HIV effects[9]. Gomisin A also suppresses liver carcinogenesis by tumor-promoting agents[10–12]. Dimethyl-4,4´-dimethoxy-5,6,5´,6´-dimethylene dioxybiphenyl-2,2´-dicarboxylate (DDB), a synthetic drug derived from dibenzocyclooctadiene lignans, was developed as a supplement for acute and chronic hepatitis patients[13–17].
In a previous study, wuweizisu C, gomisin N, and deoxyschisandrin exhibited significant neuroprotective activities against glutamate-induced neurotoxicity in primary cultures of rat cortical cells[18]. Gomisin A improved scopolamine-induced memory impairment via the enhancement of the cholinergic nervous system in mice[19]. In addition, the beneficial effect of Fructus schisandrae on cycloheximide-induced amnesia was found to be enhanced by treatment with serotonergic 5-HT2 receptor antagonists, but was attenuated by serotonergic 5-HT1A receptor agonists and cholinergic antagonists[20,21]. Schisandra chinensis fruit was accepted as a main herbal prescription for the improvement of learning performance in senescence-accelerated mice and for scopolamine-induced memory impairment in mice[22,23].
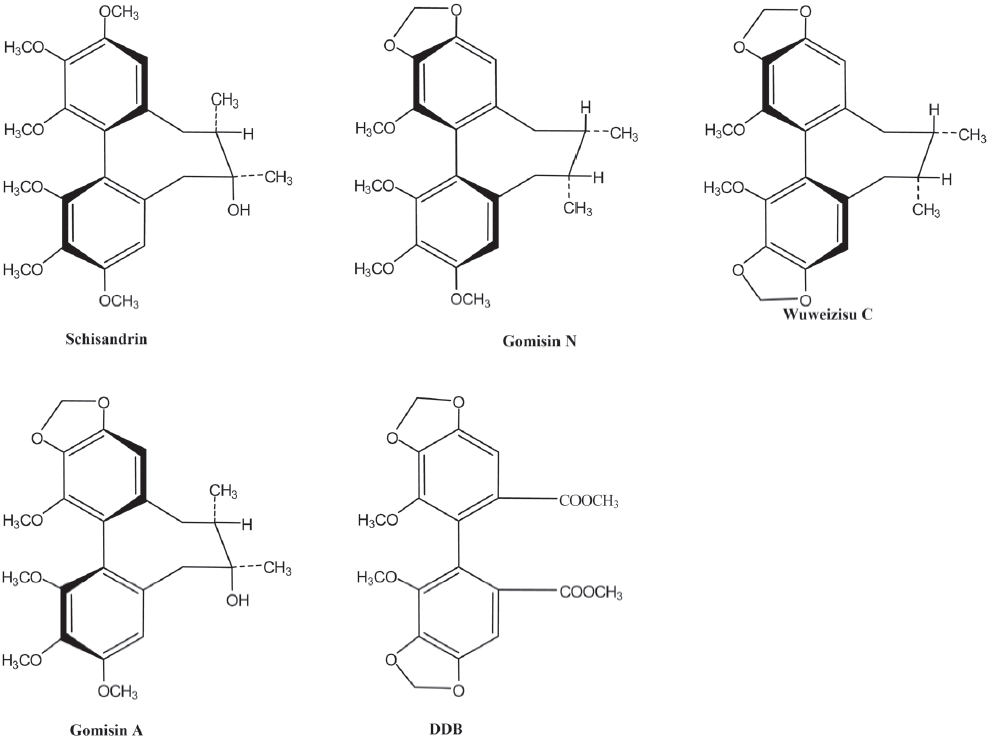
Ion channels for K+ and Cl- are involved in regulating the proliferation rate through the modulation of the membrane potential and cell volume[24]. Chlorotoxin, a putative Cl- channel-specific inhibitor, has been shown to interfere with glioma cell invasion and is now in phase II of clinical trials for the treatment of gliomas[25]. The modulation of the membrane potential plays an important role in neuronal and smooth muscle cells. However, it has been poorly investigated in glioma cells, although voltage-gated ion channels expressed in glia cells function to modulate the membrane potential and cell cycle[26]. Thus in this study, we tested the effect of 4 dibenzocyclooctadiene lignans, wuweizisu C, gomisin N, gomisin A, and schisandrin isolated and identified from Schisandra chinensis on the membrane potential in C6 glioma and PC12 neuronal cells (Figure 1) and further characterized the response with specific pharmacological inhibitors.
Materials and methods
Extraction and isolation Fruits of Schisandra chinensis were collected in September 2002 from Mujoo, Korea. A voucher specimen (Accession N
Chemical agents DiBAC4(3) was acquired from Biotium (Hayway, CA, USA). U73122 was from Biomol (Plymouth Meeting, PA, USA), and DDB was kindly provided by PharmaKing (Seoul, Korea).
Cell culture Rat C6 glioma cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium containing 10% (v/v) fetal bovine serum, 100 U/mL penicillin, 50 µg/mL streptomycin, 2 mmol/L glutamine, and 1 mmol/L sodium pyruvate at 37 °C in a humidified 5% CO2 incubator[29]. PC12 cells were maintained in RPMI-1640 containing 5% (v/v) fetal bovine serum, 15% (v/v) horse serum, 100 U/mL penicillin, 50 µg/mL streptomycin, 2 mmol/L glutamine, and 1 mmol/L sodium pyruvate at 37 °C in a humidified 5% CO2 incubator.
Measurement of membrane potential The cells were trypsin digested, sedimented, and resuspended in HEPES-buffered medium consisting of 20 mmol/L HEPES (pH 7.4), 103 mmol/L NaCl, 4.8 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 0.5 mmol/L CaCl2, 25 mmol/L NaHCO3, and 15 mmol/L glucose, and then incubated for 30 min with 5 µmol/L DiBAC4(3). Fluorescence emission from the excitation wavelength (488 nm) was measured at 530 nm every 0.1 s by a F4500 fluorescence spectrophotometer (Hitachi, Japan). The membrane potential was estimated from measurements of the fluorescence change of DiBAC-loaded cells[29].
Measurements of intracellular Ca2+ concentration Cells were trypsin digested, sedimented, resuspended in HEPES-buffered medium (20 mmol/L HEPES [pH 7.4], 103 mmol/L NaCl, 4.8 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 0.5 mmol/L CaCl2, 25 mmol/L NaHCO3, 15 mmol/L glucose, and 0.1% bovine serum albumin [fatty acid free]), and incubated for 40 min with 5 µmol/L Fura-2/AM for the Ca2+ measurement. Intracellular Ca2+ [Ca2+]i was estimated from the change in fluorescence of the Fura 2-loaded cells[30]. The fluorescence emissions at 510 nm from excitation wavelengths of 340 and 380 nm were measured every 0.1 s, and the ratio of fluorescence intensities from the 2 wavelengths (340/380) was monitored to estimate [Ca2+]i[31].
Data presentation Representative traces for the membrane potential were chosen from 3 independent experiments and are shown in Figures 2–5. The results from 3 independent experiments, shown as the percentage of the control level, are shown in Figures 2 and 5.
Results
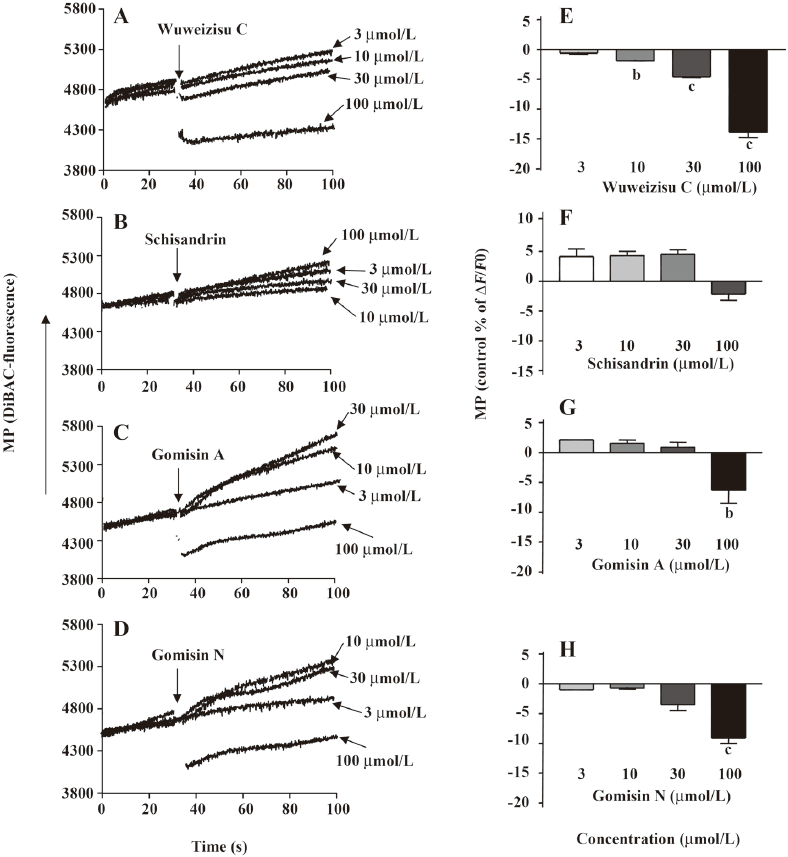
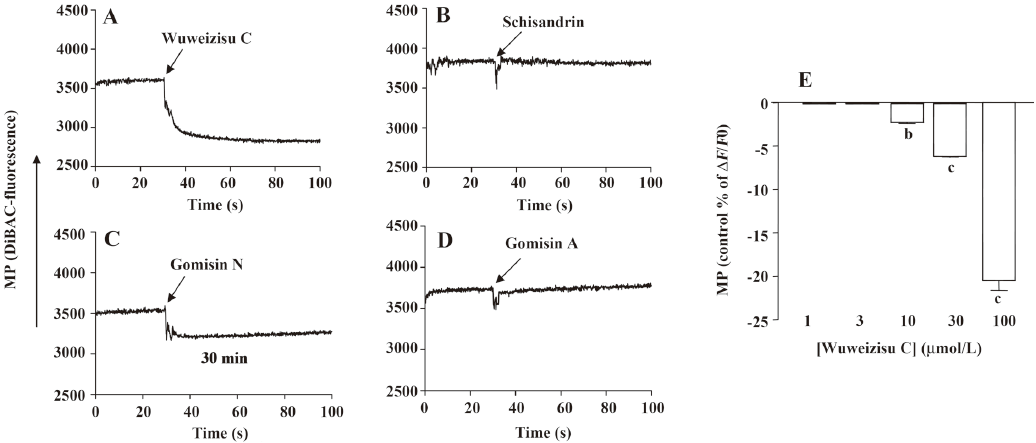
Lignans from Schisandra chinensis induce changes of membrane potential in C6 glioma cells In C6 glioma cells, wuweizisu C decreased the membrane potential in a concentration-dependent manner (Figure 2A, 2E). Gomisin N and gomisin A decreased the membrane potential by 100 μmol/L (Figure 2C, 2D, 2G, 2H). Schisandrin, however, did not change the membrane potential up to 100 μmol/L (Figure 2B, 2F). Similarly, DDB did not change the membrane potential, even at 100 μmol/L (data not shown).
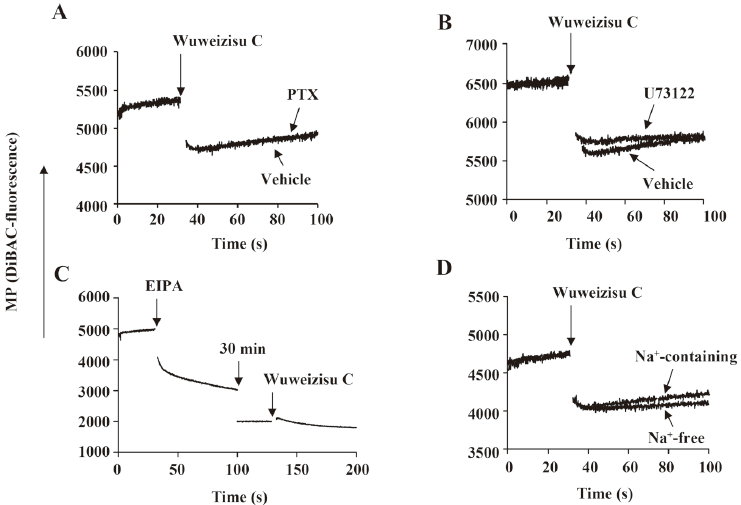
Involvement of G proteins and phospholipase C in wuweizisu C-induced membrane potential Pertussis toxin has been used to elucidate the involvement of Gi/o-type G proteins in various signaling pathways[29,32]. Thus we treated C6 glioma cells with pertussis toxin (100 ng/mL, 24 h). However, the wuweizisu C-induced decrease in the membrane potential was not blunted, suggesting no involvement of G-protein-coupled receptors coupled to Gi/o-type G proteins (Figure 3A). U73122 is a pharmacological tool to test the involvement of phospholipase C located in the plasma membrane and activated through Gq/11-protein-coupled receptors[32]. We treated C6 glioma cells with U73122 (5 μmol/L, 10 min) and found no change in the wuweizisu C-induced decrease in the membrane potential (Figure 3B).
Effects of 5-(N-Ethyl-N-isopropyl)-amiloride (EIPA) and Na+-free media on the wuweizisu C-induced membrane potential We next tested the effects of EIPA and Na+-free media on the wuweizisu C-induced change in the membrane potential. EIPA is an inhibitor of the Na+/H+ exchanger (NHE). By itself, EIPA treatment dramatically decreased the membrane potential and this decrease lasted for 30 min (Figure 3C). After 30 min of EIPA treatment, it was impossible to say whether NHE was a component of the wuweizisu C-induced decrease in the membrane potential because the resting membrane potential was decreased by the EIPA treatment. However, EIPA-sensitive NHE was definitely a regulator of the membrane potential in C6 cells[33].
When the effect of wuweizisu C on the membrane potential was measured in Na+-free medium, the wuweizisu C-induced decrease in the membrane potential was not affected by the depletion of Na+ in the extracellular medium (Figure 3D), suggesting that extracellular Na+ is not important for the membrane potential decrease by wuweizisu C in C6 glioma cells.
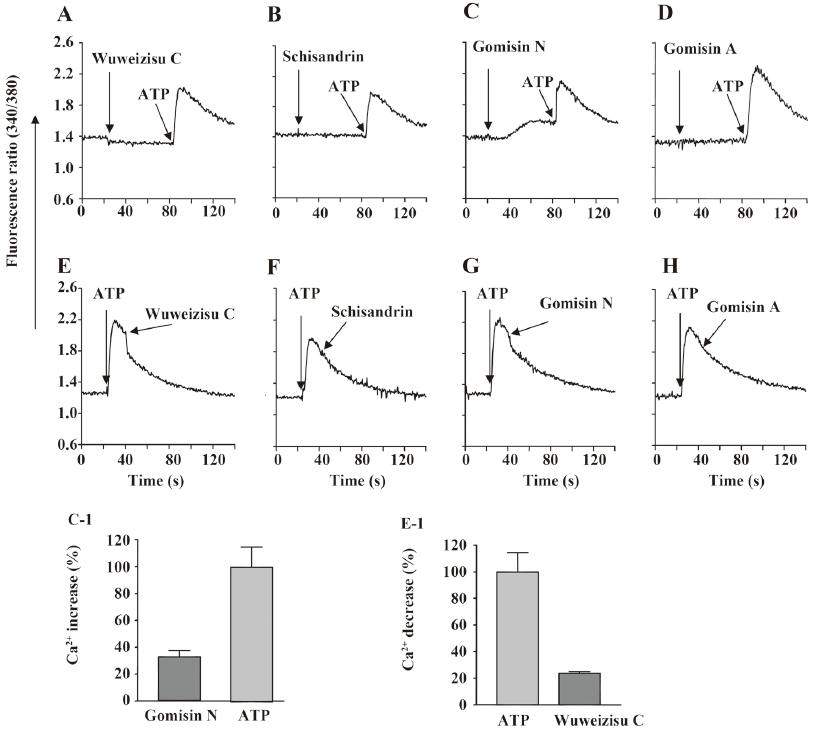
Effect of wuweizisu C on [Ca2+]i concentration We also measured changes in the [Ca2+]i concentration. By themselves, the 4 lignans did not change [Ca2+]i, with the exception of gomisin N (Figure 4C). Gomisin N slowly increased [Ca2+]i; however, the increase did not affect the following the ATP-induced [Ca2+]i increase. Wuweizisu C inhibited the ATP-induced increase of [Ca2+]i, but the increase was not inhibited by other lignans (Figure 4E-4H). The wuweizisu C-induced inhibition and gomisin N-induced increase of [Ca2+]i implied the modulation of Ca2+ homeostasis by the lignans from Schisandra chinesis.
Wuweizisu C induces a decrease in membrane potential in PC12 neuronal cells To see the effects of these 4 lignans on the membrane potential in neurons, changes in the membrane potential induced by the lignans were measured in PC12 neuronal cells. Similarly, wuweizisu C decreased the membrane potential in a concentration-dependent manner (Figure 5A, 5E). Gomisin N decreased the membrane potential only at 100 μmol/L (Figure 5C). Schisandrin and gomisin A, however, did not change the membrane potential even at 100 μmol/L (Figure 5B, 5D), suggesting differential sensitivity of PC12 neuronal cells to each lignan.
Discussion
In this study, the effects of wuweizisu C, gomisin A, gomisin N, and schisandrin, which are dibenzooctadiene lignans of Schisandra fruits, on the membrane potential were investigated in C6 glioma cells. We previously observed an increase of the membrane potential with sarcotride A and bio-active lysophospholipids, such as lysophosphatidic acid and lysophosphatidylserine in C6 glioma cells[29,33,34]. In the present study, we showed decreases of the membrane potential by wuweizisu C, gomisin N, and gomisin A. Although the precise mechanism for the decreases was not elucidated, we found independence of pertussis-sensitive G proteins, U73122-sensitive phospholipase C, and extracellular Na+. Furthermore, we found that wuweizisu C decreased the membrane potential in PC12 neuronal cells. We also found that wuweizisu C inhibits the ATP-induced increase of the [Ca2+]i concentration, and by itself, gomisin N increases the basal Ca2+ concentration. In primary-cultured rat cortical cells, the kainic acid-induced Ca2+ influx was significantly inhibited by wuweizisu C and gomisin N, supporting our observation[18].
Because schisandrin, gomisin A, and gomisin N have been shown to reverse cancer drug resistance by targeting P-glycoprotein (P-gp, ABCB1) or multidrug resistance-associated protein 1 (ABCC1)[35–41], we tested the effect of probenecid on the membrane potential in C6 glioma cells. However, probenecid (50 and 500 μmol/L) did not change the membrane potential in C6 glioma cells, excluding the possibility that wuweizisu C decreases the membrane potential through the modulation of P-gp in C6 glioma cells (data not shown).
The modulation of the membrane potential was induced by wuweizisu C, gomisin N, and gomisin A, but not by schisandrin. Three modulatory lignans have 1–2 methylenedioxy groups on dibenzocyclooctadiene, but not in the schisandrin structure. Furthermore, gomisin N and gomisin A, which have just 1 methylenedioxy group, showed a decrease of the membrane potential at a 100 μmol/L concentration. Wuweizisu C, which has 2 methylenedioxy groups, showed a concentration-dependently decrease of the membrane potential. Thus we presumed that the 2 methylenedioxy groups are important for the modulation of the membrane potential and tested DDB, which has 2 methylenedioxy groups, but not the cyclooctadiene ring. DDB did not change the membrane potential like schisandrin in C6 glioma cells (data not shown), suggesting the importance of the cyclooctadiene ring as well as the methylenedioxy groups. This idea is supported by the changes in the membrane potential in PC12 neuronal cells because gomisin N decreased the membrane potential, but gomisin A did not, showing differential responses in glioma and neuronal cells to lignans containing 1 methylenedioxy group.
In summary, we showed the decrease in the membrane potential by wuweizisu C in C6 glioma and PC12 neuronal cells and its inhibition of the ATP-induced [Ca2+]i increase. Although the precise mechanism for this decrease was not elucidated, the present study provides useful information for the elucidation of the action mechanisms of Schisandra chinensis, especially the lignans and drug development using Schisandra chinensis (fruits) or active lignans.
References
- Liu GT. Pharmacological actions and clinical use of Fructus schizandrae. Chin Med J (Engl) 1989;102:740-9.
- Cyong JC, Ki SM, Iijima K, Kobayashi T, Furuya M. Clinical and pharmacological studies on liver disease treated with Kampo herbal medicine. Am J Chin Med 2000;28:351-60.
- Ikeya Y, Taguchi H, Yosioka I, Kobayashi H. The constituents of Schizandra chinensis Baill. I. Isolation and structure determination of five new lignans, gomisin A, B, C, F and G, and the absolute structure of schizandrin. Chem Pharm Bull (Tokyo) 1979;27:1383-94.
- Lee YW, Voyksner RD, Pack TW, Cook CE, Fang QC, Ito Y. Application of countercurrent chromatography/thermospray mass spectrometry for the identification of bioactive lignans from plant natural products. Anal Chem 1990;62:244-8.
- Maeda S, Takeda S, Miyamoto Y, Aburada M, Harada M. Effects of gomisin A on liver functions in hepatotoxic chemicals-treated rats. Jpn J Pharmacol 1985;38:347-53.
- Kiso Y, Tohkin M, Hikino H, Ikeya Y, Taguchi H. Mechanism of antihepatotoxic activity of wuweizisu C and gomisin A. Planta Med 1985.331-4.
- Yamada S, Murawaki Y, Kawasaki H. Preventive effect of gomisin A, a lignan component of shizandra fruits, on acetaminophen-induced hepatotoxicity in rats. Biochem Pharmacol 1993;46:1081-5.
- Wang JP, Raung SL, Hsu MF, Chen CC. Inhibition by gomisin C (a lignan from Schizandra chinensis) of the respiratory burst of rat neutrophils. Br J Pharmacol 1994;113:945-53.
- Fujihashi T, Hara H, Sakata T, Mori K, Higuchi H, Tanaka A, et al. Anti-human immunodeficiency virus (HIV) activities of halogenated gomisin J derivatives, new nonnucleoside inhibitors of HIV type 1 reverse transcriptase. Antimicrob Agents Chemother 1995;39:2000-7.
- Yasukawa K, Ikeya Y, Mitsuhashi H, Iwasaki M, Aburada M, Nakagawa S, et al. Gomisin A inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology 1992;49:68-71.
- Miyamoto K, Wakusawa S, Nomura M, Sanae F, Sakai R, Sudo K, et al. Effects of gomisin A on hepatocarcinogenesis by 3'-methyl-4-dimethylaminoazobenzene in rats. Jpn J Pharmacol 1991;57:71-7.
- Nomura M, Nakachiyama M, Hida T, Ohtaki Y, Sudo K, Aizawa T, et al. Gomisin A, a lignan component of Schizandora fruits, inhibits development of preneoplastic lesions in rat liver by 3'-methyl-4-dimethylamino-azobenzene. Cancer Lett 1994;76:11-8.
- Park EY, Ki SH, Ko MS, Kim CW, Lee MH, Lee YS, et al. Garlic oil and DDB, comprised in a pharmaceutical composition for the treatment of patients with viral hepatitis, prevents acute liver injuries potentiated by glutathione deficiency in rats. Chem Biol Interact 2005;155:82-96.
- Akbar N, Tahir RA, Santoso WD. Effectiveness of the analogue of natural Schisandrin C (HpPro) in treatment of liver diseases: an experience in Indonesian patients. Chin Med J (Engl) 1998;111:248-51.
- Kim SN, Kim SY, Yim HK, Lee WY, Ham KS, Kim SK, et al. Effect of dimethyl-4,4'-dimethoxy-5,6,5',6'-dimethylenedio-xybiphenyl-2,2'- dicarboxylate (DDB) on chemical-induced liver injury. Biol Pharm Bull 1999;22:93-5.
- Huber R, Hockenjos B, Blum HE. DDB treatment of patients with chronic hepatitis. Hepatology 2004;39:1732-3.
- Gao H, Zhou YW. Anti-lipid peroxidation and protection of liver mitochondria against injuries by picroside II. World J Gastroenterol 2005;11:3671-4.
- Kim SR, Lee MK, Koo KA, Kim SH, Sung SH, Lee NG, et al. Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. J Neurosci Res 2004;76:397-405.
- Kim DH, Hung TM, Bae KH, Jung JW, Lee S, Yoon BH, et al. Gomisin A improves scopolamine-induced memory impairment in mice. Eur J Pharmacol 2006;542:129-35.
- Hsieh MT, Wu CR, Wang WH, Lin LW. The ameliorating effect of the water layer of Fructus Schisandrae on cycloheximide-induced amnesia in rats: interaction with drugs acting at neurotransmitter receptors. Pharmacol Res 2001;43:17-22.
- Hsieh MT, Tsai ML, Peng WH, Wu CR. Effects of Fructus schizandrae on cycloheximide-induced amnesia in rats. Phytother Res 1999;13:256-7.
- Nishiyama N, Chu PJ, Saito H. An herbal prescription, S-113m, consisting of biota, ginseng and schizandra, improves learning performance in senescence accelerated mouse. Biol Pharm Bull 1996;19:388-93.
- Kang SY, Lee KY, Koo KA, Yoon JS, Lim SW, Kim YC, et al. ESP-102, a standardized combined extract of Angelica gigas, Saururus chinensis and Schizandra chinensis, significantly improved scopolamine-induced memory impairment in mice. Life Sci 2005;76:1691-705.
- Rouzaire-Dubois B, Milandri JB, Bostel S, Dubois JM. Control of cell proliferation by cell volume alterations in rat C6 glioma cells. Pflugers Arch 2000;440:881-8.
- Weaver AK, Bomben VC, Sontheimer H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 2006;54:223-33.
- Higashimori H, Sontheimer H. Role of Kir4.1 channels in growth control of glia. Glia 2007;55:1668-79.
- Choi YW, Takamatsu S, Khan SI, Srinivas PV, Ferreira D, Zhao J, et al. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J Nat Prod 2006;69:356-9.
- Avula B, Choi YW, Sirinivas PV, Khan IA. Quantitative determination of lignan constituents from Schisandra chinensis by liquid chromatography. Chromatographia 2005;61:515-8.
- Lee YK, Im DS. Distinct effects of lysophospholipids on membrane potential in C6 glioma cells. J Appl Pharmacol 2006;14:25-9.
- Chang YJ, Lee YK, Lee EH, Park JJ, Chung SK, Im DS. Structure–activity relationships of dimethylsphingosine (DMS) derivatives and their effects on intracellular pH and Ca2+ in the U937 monocyte cell line. Arch Pharm Res 2006;29:657-65.
- Yun MR, Okajima F, Im DS. The action mode of lysophosphatidylcholine in human monocytes. J Pharmacol Sci 2004;94:45-50.
- Im DS, Fujioka T, Katada T, Kondo Y, Ui M, Okajima F. Characterization of sphingosine 1-phosphate-induced actions and its signaling pathways in rat hepatocytes. Am J Physiol 1997;272:G1091-9.
- Lee YK, Kim K, Kim HL, Sacket SJ, Han M, Jo JY, et al. Lysophosphatidylserine increases membrane potentials in rat C6 glioma cells. Arch Pharm Res 2007;30:1096-101.
- Lee YK, Liu Y, Jung JH, Im DS. Effect of sarcotride A on membrane potential in C6 glioma cells. J Appl Pharmacol 2006;14:110-3.
- Pan Q, Lu Q, Zhang K, Hu X. Dibenzocyclooctadiene lignans: a class of novel inhibitors of P-glycoprotein. Cancer Chemother Pharmacol 2006;58:99-106.
- Qiangrong P, Wang T, Lu Q, Hu X. Schisandrin B—a novel inhibitor of P-glycoprotein. Biochem Biophys Res Commun 2005;335:406-11.
- Li L, Lu Q, Shen Y, Hu X. Schisandrin B enhances doxorubicin-induced apoptosis of cancer cells but not normal cells. Biochem Pharmacol 2006;71:584-95.
- Li L, Pan Q, Sun M, Lu Q, Hu X. Dibenzocyclooctadiene lignans: a class of novel inhibitors of multidrug resistance-associated protein 1. Life Sci 2007;80:741-8.
- Wan CK, Zhu GY, Shen XL, Chattopadhyay A, Dey S, Fong WF. Gomisin A alters substrate interaction and reverses P-glycoprotein-mediated multidrug resistance in HepG2-DR cells. Biochem Pharmacol 2006;72:824-37.
- Fong WF, Wan CK, Zhu GY, Chattopadhyay A, Dey S, Zhao Z, et al. Schisandrol A from Schisandra chinensis reverses P-glycoprotein-mediated multidrug resistance by affecting P-gp-substrate complexes. Planta Med 2007;73:212-20.
- Sun M, Xu X, Lu Q, Pan Q, Hu X. Schisandrin B: a dual inhibitor of P-glycoprotein and multidrug resistance-associated protein 1. Cancer Lett 2007;246:300-7.