IL-1beta sensitizes rat intervertebral disc cells to Fas ligand mediated apoptosis in vitro
Introduction
Low back pain is a leading cause of morbidity. It is estimated that about 70% of the population will experience low back pain during their lives[1]. In recent years, intervertebral disc (IVD) disorders and age-related degeneration have been significant contributors to low back pain and spine-related disability. There is great interest in understanding the complex pathogenesis of IVD diseases and recent reports of the existence of apoptotic cells in IVD have provided a new insight into the pathophysiology of IVD degeneration[2–8].
Fas/FasL system-mediated apoptosis is thought to play an important role in the loss of disc cells that leads to diminished generation, organization, and repair of the extracellular matrix in the herniated lumbar disc tissues. The disc cells, after herniation, undergo apoptosis via autocrine or paracrine FasL mechanisms by the disc cells themselves[2,5–8]. Fas (CD95) and Fas ligand (FasL) belong to the TNF family. The binding of FasL with Fas triggers the formation of the death-inducing signaling complex by recruiting an adaptor molecule Fas-associated death domain (FADD) to the cytoplasmic tail of Fas (C-terminal region). The subsequent autocatalytic activation of a downstream cascade of caspases leads to the cleavage of specific substrates and thus, the activation of the apoptotic executioners[9–12].
Herniated IVD tissue has been shown to produce pro-inflammatory cytokines, including matrix metalloproteinases (MMPs), interleukin (IL)-1β, interleukin-1α, interleukin-6, tumor necrosis factor-alpha (TNF-α), nitric oxide(NO), and prostaglandin E2 (PGE-2)[13-18].
We proposed that normal disc cells can upregulate apoptosis in an inflammation microenvironment attributed to the production of a large number of inflammatory cytokines in the disc degeneration process. Therefore, the goal of the current study was to investigate the apoptotic effect and the Fas gene expression on cultured rat IVD cells which were stimulated by Fas ligand and IL-1β.
Materials and methods
Primary disc cell isolation All cell culture supplies were purchased from Gibco BRL (Gaithersburg, MD, USA) unless otherwise noted. Lumbar IVD (L3 to L6) from Sprague–Dawley rats (aged 3 months, male, 455±29 g in weight) were harvested immediately in a sterile environment after they were killed. The nucleus pulposus (NP) was removed and the inner annulus, including the transition zone (TZ), was separated through an operating microscope. The determination between the outer and inner annulus was based on the amount of hydrated ground substance between the lamellae. The outer annulus is a dense, fibrous tissue, with little space between the oriented lamellar layers. The inner annulus, including the TZ, contains more ground substance which causes the lamellae to distend and become less distinct and organized. For most discs, the outer one-third of the annulus is designated as the outer annulus, and the inner two-thirds are designated as the inner annulus (Figure 1).

The inner annulus fibrosus (IAF), including the TZ between the annulus and the nucleus, was dissected and placed in a humidified incubator with 5% CO2 at 37 ºC in Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium (DMEM/F-12) with 10% fetal bovine serum (FBS) (Hyclone, Utah, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin for 8 h. To isolate the cells, the disc tissues in the DMEM/F-12 medium were digested with 0.25% trypsin
Cell culture in selected concentrations of recombinant rat IL-1β or the recombinant rat Fas ligand. When the primary cell culture became confluent, the cells were trypsinized and subcultured into 6-well plates at 3×105cells/well. The cells were cultured in DMEM/F-12 medium with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37 ºC. When the confluence in each well was over 80%, the medium was replaced with medium containing 1% FBS without penicillin and streptomycin. There were 5 treatment groups. In 3 treatment groups, the disc cells were cultured in the medium (1% FBS) for 8 h. The medium was refreshed and 10 ng/mL IL-1β (Cytolab/Peprotech Asia, Rehovot, Israel), 5 ng/mL FasL (R&D Systems, Minneapolis, MN, USA), and 20 ng/mL FasL was respectively added to the medium (1% FBS); the cells were cultured for up to another 24 h. In the other 2 treatment groups (10 ng/mL IL-1β+5 ng/mL FasL, and 10 ng/mL IL-1β+20 ng/mL FasL), the cells were pretreated with 10 ng/mL IL-1β for 8 h in the medium (1% FBS). The medium was then refreshed and FasL (5, and 20 ng/mL) was respectively added to the medium (1%FBS); the cells cultured for up to another 24 h. Cultures without addition of IL-1β or FasL acted as the controls. After 32 h, the cells were double stained with Annexin V–fluorescein isothiocyanate (Annexin V–FITC) and propidium iodide (PI) (Bender MedSystems, Vienna, Austria), and the cells were harvested for RNA extraction.
Flow cytometry(FCM) Apoptosis was determined by staining cells with both Annexin V–FITC and PI, according to the manufacturer’s instructions. Annexin V–FITC is used to quantitatively determine the percentage of cells undergoing apoptosis. It relies on the property of cells to lose membrane asymmetry in the early phase of apoptosis. In apoptotic cells, the membrane phospholipid phosphatidylserine is translocated from the inner leaflet of the plasma membrane to the outer leaflet, thereby exposing phosphatidylserine to the external environment. Cells that were positively stained with Annexin V–FITC and negatively stained for PI were considered apoptosis. Cells that were positively stained for both Annexin V–FITC and PI were considered necrosis[19,20]. To quantitate apoptosis, the cells were washed with cold phosphate-buffered saline solution and then resuspended in binding buffer (10 mmol/L HEPES (N-2-hydroxyethylpipera-zine-N‚-2-ethanesulphonic acid)/NaOH [pH 7.4], 140 mmol/L NaCl, and 2.5 mmol/L CaCl2). The cells were stained with 5 µL Annexin V–FITC and 10 µL PI and then analyzed with EpicsAltra (Beckman Coulter, CA, USA) FCM.
RT-PCR analysis of Fas gene transcription Total RNA was isolated from the disc cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacture’s directions. Single-strand cDNA templates were prepared from 2 µg total RNA using oligo (dT)18 and RevertAid M-MuLV reverse transcriptase (Fermentas, Vilnius, Lithuania). Specific cDNA were then amplified by PCR using the following primers (Sangon, Shanghai, China): Fas sense primer: 5’-GCATCTTTGAGGGTTTGGA-3’, antisense primer: 5’-CATTTGGTGTTGCTGGTTC-3’ and GAPDH sense primer: 5’-ACCACAGTCCATGCCATCAC-3’, antisense primer: 5’-TCCACCACCCTGTTGCTGTA-3’. PCR amplification (PCR System 2700, PE, CA, USA) from cDNA was performed in a final volume of 50 µL containing 15 mmol/L MgCl2, 1.25 U Takara Taq, and 0.3 µmol/L specific primers (TaKaRa, Dalian, China). The cycling conditions were: denaturation at 94 °C for 30 s, annealing (Fas 50 °C, GAPDH 58°C) for 30 s, and elongation at 72 °C for 60 s. The optimum cycle number was 30 cycles for Fas and 25 cycles for GAPDH. All PCR products were determined by 2% agarose gel electrophoresis by ethidium bromide staining and visualized by UV transillumination. Gel images were analyzed by densitometry using Scion Image (Scion Corp, Frederick, MD, USA). Fas gene expression data are presented as normalized to GAPDH expression.
Statistical analysis All experiments were performed at least 3 times to ensure consistency. SPSS 11.0 software (Chicago, IL, USA) was used for the statistics. Data were compared using unpaired 2-tailed Student’s t-test analysis, with a P-value of 0.05 or less considered significant.
Results
Establishment of cultures in monolayer The primary cells from the IAF, including the TZ of rat lumbar IVD, became confluent after 9 d in the monolayer. Then the primary cells were trypsinized and subcultured into 6-well plates with 3×105 cells/well. The first passage cells displayed a uniform, rounded, chondrocyte-like morphology and achieved 80% confluence after 7 d.
Evaluation of apoptosis When treated and cultured for 32 h, the apoptosis of the 6 groups was determined by double staining with Annexin V–FITC and PI. The apoptotic ratio of rat IAF and TZ cells were calculated as a percentage of apoptotic cells/total cells (Table 1). Compared with the control group, FasL (20 ng/mL), IL-1β (10 ng/mL)+FasL (5 ng/mL), and IL-1β (10 ng/mL)+FasL (20 ng/mL) induced significant apoptosis of the disc cells (P<0.01). Apoptosis was also induced by FasL 5 ng/mL (P<0.05); whereas, apoptosis was not induced by IL-1β (10 ng/mL) (P>0.05). IL-1β (10 ng/mL) enhanced the apoptosis-inducing effects of FasL (5 ng/mL) and FasL (20 ng/mL) in disc cells (Figure 2, P<0.01).

Full table

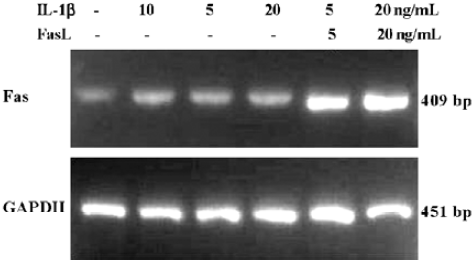
Transcription of Fas When treated and cultured for 32 h, RNA was extracted from the monolayer-cultured rat disc cells. RT-PCR was used for determining the transcription of the Fas gene. The Fas gene was transcripted in the 5 treatment and control groups (Figure 3). And the transcription levels of the Fas gene in the 5 treatment groups were approximately 1.2–2.1-fold greater than the control group (respectively, P<0.05). Additionally, group of IL-1β (10 ng/mL)+FasL (5 ng/mL) compared with group of FasL (5 ng/mL) and group of IL-1β (10 ng/mL)+FasL (20 ng/mL) compared with group of FasL (20 ng/mL) significantly upregulated transcription of Fas (respectively, P<0.01).
Discussion
Fas is a membrane-bound receptor that is activated by the binding of FasL and results in programmed cell death/apoptosis. Park et al[6] reported the effect of Fas on disc cells in human herniated disc tissues and found that the percentage of Fas-positive cells correlated significantly with patients’ age, but not with the degree of disc degeneration on magnetic resonance imaging. Wang et al[8] also found that post-operative samples had an increased number of Fas-positive cells in rat cervical degenerative disc models. Anderson et al[21,22] detected a high expression of Fas following both annular laceration in a rabbit model and fibronectin fragment coculturing with rabbit IVD cells in vitro. Fas is widely expressed in numerous different cell types throughout the body, whereas Fas ligand expression appears to be more restricted. The expression of Fas ligand in disc cells could be detected in developing embryos[23], degenerative discs[7], normal discs[24], and scoliotic discs[25]. Intervertebral discs with their extensive extracellular matrix are largely avascular tissues and display anatomically isolation from the hosts’ immune system. Many studies have demonstrated that Fas ligand should play a key role in the potential molecular mechanism to maintain immune privilege of the disc[7,24]. However, in degenerative discs[7] and scoliotic discs[25], Fas ligand had a close relationship with the apoptosis of disc cells.
The inflammatory cytokine IL-1 plays an important role in disc degeneration. IL-1 has been shown to increase the synthesis of matrix-degrading enzymes (MMP-2, MMP-3, MMP-13, and ADAMTS-4 (A Disintegrin-like and metallo-protease with thrombospondin motifs 4) and to decrease the synthesis of proteoglycan, collagen I and collagen II, and to induce the expression of IL-6, cyclooxy-genase-2, stromolysin-1, and PGE2[26–30]. IL-1β can also induce the production of endogenous IL-1β by disc cells in vitro. There is evidence to support that the positive feedback loop of IL-1β exists in degenerative disc cells which upregulate the production of mediators and thus can cause the cessation of symptoms in intervertebral disc herniation[30]. Our study demonstrated that FCM found no significant apoptosis after the disc cells were treated with IL-1β (10 ng/mL) for 24 h, however, the apoptotic rate could not deny the changes which occurred to the disc cells. The effect of IL-1β on disc degeneration has been unknown until now.
This study is the first to document normal disc cells in vitro response to FasL with/without IL-1β pre-treatment. Disc cells with IL-1β pre-treatment have a significant apoptotic rate compared with control and disc cells without IL-1β pre-treatment. This implies that the sensitivity of intervertebral discs to FasL increased after IL-1β treatment, which led to a high apoptotic rate at a low level of FasL in normal disc cells.
The present study, using RT-PCR, demonstrates that the transcription of Fas in rat lumbar disc cells increased significantly in the 5 treatment groups. It is important to note that we detected the transcription of Fas on control cells using RT-PCR. Park et al[6] and Wang et al[8] showed a similar result by means of immunohistochemistry, but Anderson et al[22] reported no apparent transcription of Fas on control discs based on the RT-PCR result.
There are 3 limits of the current study. One is that rat disc cells only from the inner annulus fibrosus and TZ were used, because rat NP primary cells could not proliferate and disappeared after 3 weeks. The second limit was that our study stimulated disc cells with IL-1β only for 24 h. It is necessary to prolong the observation time to study whether IL-1β can significantly induce apoptosis of disc cells. The third limit was that we cultured the disc cells in the monolayer, which can not completely represent the cells in vivo, so it is necessary to culture disc cells in a 3-D culture system.
In conclusion, the results of this study showed that the apoptotic rate of disc cells pretreated with IL-1β increased in response to FasL in vitro and provided insights into understanding the Fas/FasL system-mediated apoptosis in disc cells which would be enhanced due to the inflammation factor in degenerative discs.
References
- Macfarlane GJ, Thomas E, Croft PR, Papageorgiou AC, Jayson MI, Silman AJ. Predictors of early improvement in low back pain amongst consulters to general practice: the influence of pre-morbid and episode-related factors. Pain 1999;80:113-9.
- Gruber HE, Hanley EN Jr. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine 1998;23:751-7.
- Gruber HE, Norton HJ, Hanley EN Jr. Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine 2000;25:2153-7.
- Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine 1998;23:2493-506.
- Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine 2000;25:1477-83.
- Park JB, Kim KW, Han CW, Chang H. Expression of Fas receptor on disc cells in herniated lumbar disc tissue. Spine 2001;26:142-6.
- Park JB, Chang H, Kim KW. Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine 2001;26:618-21.
- Wang YJ, Shi Q, Lu WW, Cheung KC, Darowish M, Li TF, et al. Cervical intervertebral disc degeneration induced by unbalanced dynamic and static forces: a novel in vivo rat model. Spine 2006;31:1532-8.
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991;66:233-43.
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993;75:1169-78.
- Beutler B, van Huffel C. Unraveling function in the TNF ligand and receptor families. Science 1994;264:667-8.
- Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med 1995;182:1777-83.
- Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine 1996;21:271-7.
- Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine 2002;27:911-7.
- Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br 84:196-201.
- Burke JG, Watson RW, Conhyea D, McCormack D, Dowling FE, Walsh MG, et al. Human nucleus pulposis can respond to a pro-inflammatory stimulus. Spine 2003;28:2685-93.
- Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine 2005;30:44-53.
- Yoshida M, Nakamura T, Sei A, Kikuchi T, Takagi K, Matsukawa A. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin-1beta, and monocyte chemoattractant protein-1 immediately after herniation: an experimental study using a new hernia model. Spine 2005;30:55-61.
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phospha-tidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995;184:39-51.
- Zhang G, Gurtu V, Kain SR, Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques 1997;23:525-31.
- Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, et al. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine 2002;27:1291-6.
- Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine 2005;30:1242-6.
- Inui Y, Nishida K, Doita M, Takada T, Miyamoto H, Yoshiya S, et al. Fas-ligand expression on nucleus pulposus begins in developing embryo. Spine 2004;29:2365-9.
- Takada T, Nishida K, Doita M, Kurosaka M. Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine 2002;27:1526-30.
- Chen B, Fellenberg J, Wang H, Carstens C, Richter W. Occurrence and regional distribution of apoptosis in scoliotic discs. Spine 2005;30:519-24.
- Rannou F, Corvol MT, Hudry C, Anract P, Dumontier MF, Tsagris L, et al. Sensitivity of anulus fibrosus cells to interleukin 1 beta. Comparison with articular chondrocytes. Spine 2000;25:17-23.
- Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine 2000;25:166-9.
- Shen B, Melrose J, Ghosh P, Taylor F. Induction of matrix metalloproteinase-2 and -3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1beta: a potential pathway of disc degeneration. Eur Spine J 2003;12:66-75.
- Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 2005;7:R732-45.
- Jimbo K, Park JS, Yokosuka K, Sato K, Nagata K. Positive feedback loop of interleukin-1beta upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J Neurosurg Spine 2005;2:589-95.
