Salvicine, a novel topoisomerase II inhibitor, exerts its potent anticancer activity by ROS generation1
Introduction
Topoisomerase II (Topo II) is essential for DNA metabo-lism, where it acts to adjust the topology of DNA during transcription, replication, recombination, repair and mito-sis[1]. Topo II have been validated as clinically important targets for cancer chemotherapy, and their inhibitors are central components in many therapeutic regimens[2,3]. These Topo II-targeting agents can be divided into two categories according to their mechanisms of action: Topo II poisons and catalytic inhibitors. Topo II poisons are able to stabilize the reversible covalent Topo II-DNA complex termed the cleavage complex, while catalytic inhibitors act on the other steps in the catalytic cycle without trapping the covalent complex. Although Topo II poisons are among the frequently used regimens in the clinical treatment of human malign-ancies, there are still some limitations such as dose-limiting toxicities and drug resistance leading to treatment failure after initial effective therapy. Moreover, drugs originating from different chemical families, which share a common cellular target, generally exhibit different spectra of anticancer activity. Consequently there is an increasing interest focusing on searching and developing anticancer agents targeting human Topo II.
Salvicine is a novel diterpenoid quinone compound obtained by structural modification of a natural product lead isolated from Salvia prionitis Hance (Labiatae) with potent growth inhibitory activity against a wide spectrum of human tumor cells in vitro[4] and in mice bearing human tumor xenograft[5] (Yuan S, et al, unpublished data). Salvicine has also been found to have a profound cytotoxic effect on multidrug-resisitant (MDR) cells. Moreover, Salvicine significantly reduced the lung metastatic foci of MDA-MB-435 orthotopic xenograft with no obvious inhibition on primary tumor growth. These results demonstrate that salvicine is a promising anticancer drug candidate and is in phase II clinical trials in China.
Salvicine has emerged as a novel Topo II inhibitor with distinct modes of action, which are dependent on ROS generation. This review will focus on the effects and mechanisms of salvicine as a novel Topo II inhibitor as well as its distinguished anticancer activity in different systems.
Salvicine is a novel Topo II poison with distinct mode of action
Salvicine was found to inhibit the activity of Topo II in a routine screen with an approximate IC50 value of 3 µmol/L in kDNA decatenation assays. Similar results were attained by Topo II-mediated supercoiled DNA relaxation assay[7]. Salvicine acted as a Topo II poison through its marked enhancement effect on Topo II-mediated DNA double-strand breaks as observed in the DNA cleavage assay without intercalating into DNA[6]. In contrast, no inhibitory activity was observed against the catalytic activity of TopoI[6]. Consistently, cytotoxicities of salvicine to parent (JN394) and TOP1 deleted (JN394top1-) yeast cells are at the same level[7]. Salvicine displays high activity against JN394t2-1 cells at 25°C where the top2-1 protein shows wild type activity, while no growth inhibition was observed at the semi-permissive temperature of 30°C in the concentration range of interest[7]. Furthermore, JN394t2-5 cells, which express top2-5 mutant allele are highly resistant to salvicine and etoposide (VP16)[7]. These results indicate that Topo II is the primary cellular target of salvicine and confirm that salvicine kills yeast cells mainly by trapping the DNA-Topo II cleavage complex.
Salvicine elicites ROS and acts on multi-steps in the catalytic cycle of Topo II By dissecting individual steps of the catalytic cycle of Topo II, the mechanism by which salvicine inactivates Topo II was found to be different to that of other anti-Topo II agents. Salvicine greatly promotes Topo II-DNA binding and inhibits pre- and post-strand Topo II-mediated DNA relegation without interference with the forward cleavage steps[6]. Moreover, molecular modeling studies predicted that salvicine binds to the ATP pocket in the ATPase domain and superimposes on the phosphate and ribose group[8]. Consistently, salvicine exhibits higher affinity for the ATPase domain of human Topo IIa than ADP and ATP in the surface plasmon resonance binding assays and inhibits ATP hydrolysis catalyzed by this domain[8]. ATP competitively and dose-dependently blocks the interactions between salvicine and the ATPase domain of Topo IIα, indicating that salvicine shares a common binding site with ATP and functions as an ATP competitor[8]. Thus, salvicine is the first reported non-intercalative eukayotic Topo II poison that binds to the ATP-binding pocket of Topo IIα. It is noteworthy that inhibition of Topo II activity was abrogated by GSH(Cai Y, et al, unpublished data), which is a ROS scavenger, suggesting that the inhibitory effect of salcivine on Topo II might due to ROS generation. Together, salvicine emerged as a novel Topo II poison with a distinct mode of action with ROS generation, competitively binding to ATP pocket, promoting Topo II-DNA binding and inhibiting Topo II-mediated DNA relegation (Figure 1).
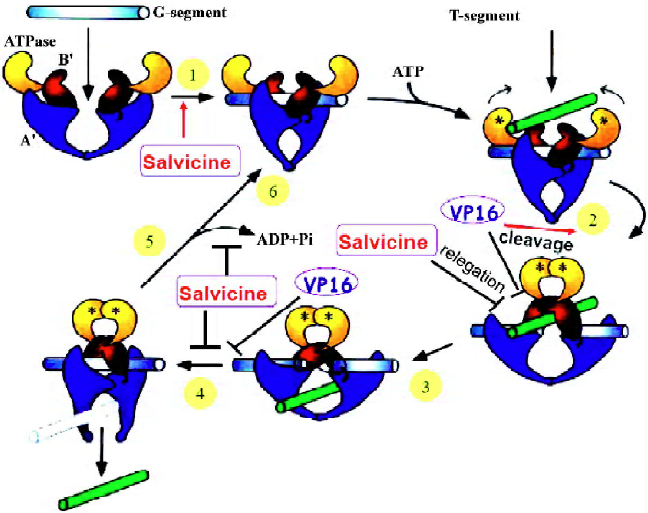
ROS-dependent and Topo II-mediated DNA damage response induced by salvicine
It is well accepted that Topo II poisons exert antipro-liferative activity by inducing DNA double stand beaks, which may in turn trigger DNA damage response cascades and ultimately apoptosis. It is notable that salvicine stimulates intracellular ROS production and subsequently elicits DSBs[9]. N-acetyl cysteine (NAC), an antioxidant, effectively attenuates the salvicine-induced ROS enhancement and also DNA DSBs. Moreover, NAC abrogates salvine-induced Topo II-DNA cleavable complexes formation and the growth inhibition of salvicine-treated JN394top2-4 yeast cells, collectively indicating that Topo II is a target of the salvicine-induced ROS[9]. Heat treatment that reversed the salvicine-trapped DNA-Topo II cleavage complex reversed the accumulation of DNA DSB as well, demonstrating that salvicine-induced DNA damage is triggered by ROS and mediated by Topo II. The breakage and/or reunion reaction of DNA Topo II can be interrupted by DNA intercalators (eg doxorubicin), enzyme binders (eg etoposide), DNA lesions (eg abasic sites), or oxidative stress to produce Topo-mediated DNA damage[10,11]. Salvicine structurally contains quinone, a chemically active moiety. Most of the quinone-containing anticancer drugs are believed to stimulate ROS as part of their anti-tumor activities or toxicities[10,12]. Accordingly, NAC attenuated salvicine-induced apoptosis and cytotoxicity in MCF-7 cells[9]. Thus, salvicine generates ROS that modulates Topo II-mediated DNA damage, contributing to the comprehensive biological consequences of salvicine treatment, such as DNA DSBs, apoptosis, and cytotoxicity in tumor cells.
Salvicine selectively induces DNA damage in the c-myc p2 promoter region Salvicine induces DNA strand breaks in human promyelocytic leukemia HL-60 cells and breast cancer MCF-7 cells [13,14]. DNA damage correlates well with cell growth inhibition, suggesting that Topo II is the primary cellular target of salvicine, which is also demonstrated by the study using a yeast genetic system[7]. DNA damage induced by brief exposure to salvicine could be partially reversed, but early DNA breaks triggered the process of apoptosis[13]. Preferential damage in the P2 promoter region of the oncogene c-myc was detected, whereas no obvious DNA damage was found in the 3' region of the same gene in both HL-60 and MCF-7 cells[13,14]. Salvicine induces a dose-dependent decrease in c-myc gene transcription, concomitant with an increase in c-jun expression[13,14]. It appears possible that DNA damage within such genomic regions is an early event, which could lead to growth inhibition mediated by alterations of the expression of selected proliferation regulatory genes, such as c-myc, c-jun, and ultimately cell death.
Salvicine disrupted the catalytic subunit of DNA-dependent protein kinase DNA DSBs induced by salvicine activates Ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) kinases and phosphorylastion of histone H2AX in lung carcinoma A549 cells (Zhang Y, et al, unpublished data), which are well documented in DSB-induced cellular responses[15]. Unexpectedly, salvicine selectively downregulates the protein levels of the catalytic subunit of DNA-dependent protein kinase (DNA-PK[CS]) but not the Ku70 and Ku86 subunit[9]. Salvicine treatment also reduces the kinase activity of DNA-PK in MCF-7 cells, which might be due to the reduction of the (DNA-PK[CS]) protein[9]. DNA-PK is composed of DNA-PKcs of approximately 450 kDa and two smaller Ku subunits (Ku70 and Ku86), is a critical component of non-homologous end joining (NHMJ) pathway[16], which is the predominant pathway for DSB repair (including Topo II-mediated DNA damage repair) in mammals[17]. Thus, salvicine simultaneously damages DNA and disrupts the DNA repair pathway, which could enhance its therapeutic effectiveness and overcome the resistance caused by DNA repair[18]. NAC pretreatment abrogates the effects of salvicine on the protein level and the activity of DNA-PK, indicating that ROS generation is involved in the salvicine-induced DNA damage and repair (Figure 2). The effects of salvicine-induced ROS on Topo II and DNA-PK give new insights into the diverse biological activities of ROS.
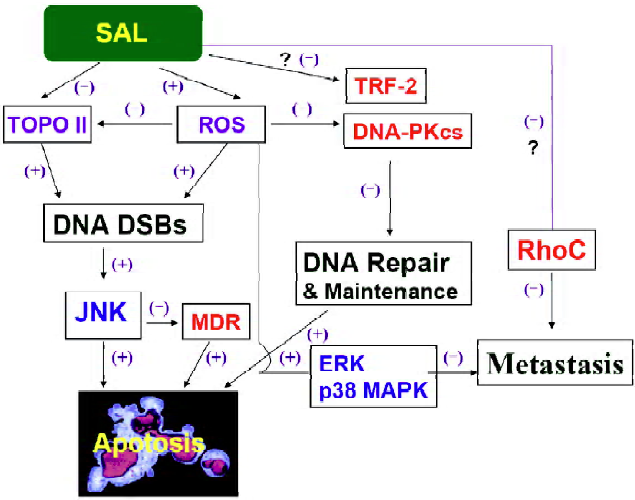
Salvicine induced telomere erosion and down-regulated Telomere repeat binding factor 2 Except for genomic DNA damage, salvicine has been shown to induce telomere erosion and to downregulate the activity of telomerase[19,20]. Salvicine treatment resulted in apoptosis and down-regulation of telomerase activity in a time- and concentration-dependent manner in HL-60 cell[19]. Further study proved that telomerase inhibition by salvicine is an early event in human lung carcinoma A549 cells[20]. Though salvicine, VP-16, mitomycin C, cisplatin and adriamycin induce telomere erosion in A549 cells, only salvicine downregulates the activity of telomerase after high concentration and short exposure[20]. Telomere repeat binding factor 2 (TRF2) has been increasingly recognized to be involved in DNA damage response and telomere maintenance[21]. Salvicine led to disruption of TRF2, independently of either its transcription or proteasome-mediated degradation (Zhang Y, et al, unpublished data). TRF2 protein was found to protect both telomeric and non-telomeric DNA from salvicine-induced damage by overexpressing the full-length trf2 gene (Zhang Y, et al, unpublished data). Together, salvicine could induce DNA damage both in genomic DNA and telomere and, at the same time, disrupt TRF2, which maintains the integrity of DNA (Figure 2).
Salvicine displays potent anticancer activity
Salvicime possesses potent antitumor activity in vitro and in vivo Salvicine displayes potent growth inhibitory activity against a panel of human tumor cells in vitro and in mice bearing human tumor xenografts[4,5]. Salvicine is as cytotoxic as VP-16 and weaker than VCR in three leukemia cell lines measured by microculture tetrazolium (MTT) assays after 72-h treatment[4]. Salvicine is over 5.41- and 4.15-fold more potent than VCR and VP-16 against 12 lines of solid tumor cells[4]. Particularly, Salvicine presents better activities against gastric and lung carcinoma cells. Moreover, the antitumor effect of salvicine was found to be associated with its ability to induce apoptosis in K-562 and SGC-7901 cells[22]. The anticancer activity of salvicine was also evaluated in animal models. Salvicine possesses a significant antineoplastic activity against murine S-180 sarcoma and Lewis lung cancer, and human lung adenocarcinoma xenografts A-549 and LAX-83[5]. Consistent with the results from the in vitro study, salvicine displays significant inhibition on lung and gastric adeocarcinoma including A-549, SPC-A4, SGC-7901, MKN-28 and MKN-45 xenografts with the optimal T/C value of 39.4%, 48.5%, 40.0%. 58.6% and 55.7%, respectively, while salvicine has no growth inhibitory effects on IBC, BEL-7402, HO8910 and HCT116 nude mice xenografts (Yuan S, et al, unpublished data). Phase 2 clinical trials demonstrated that salvicine is well-tolerated by the patients with little side effects and toxicities (unpublished data).
Salvicine overcomes multidrug resistance and down-regulates P-glycoprotein by JNK activation MDR is considered to be an important impediment to the effective chemotherapy of cancer. Therefore, it is noteworthy that salvicine is able to overcome the MDR caused by P-gp overexpression. Salvicine effectively kills tumor cells overexpressing P-gp with IC50 values of 1.55 µmol/L for K562/A02 cells, 4.50 µmol/L for KB/VCR cells, and 1.40 µmol/L for MCF-7/ADM cells, close to those for their corresponding parental cell lines: 0.87 µmol/L for K562 cells, 2.26 µmol/L for KB cells, and 2.61 µmol/L for MCF-7 cells[23]. The mean resistance factor for salvicine is 1.42, which is much lower than that of vincristine (344.35), doxorubicin (233.19) and etoposide (71.22)[23]. Salvicine induces similar levels of apoptosis in MDR K562/A02 and parental K562 cells, promising its activity against MDR. Unlike other MDR modulators that inhibit the drug efflux by P-gp, salvicine downregulates mdr-1 and P-gp expression in K-562/A02 MDR cells[23]. Further study indicated that the transcription factor c-Jun activation induced by salvicine repressed mdr-1 gene expression[24]. Salvicine suppresses mdr-1 expression in MDR cells and promotes c-jun expression in both MDR and parental K562 cells. Moreover, enhanced c-jun expression precedes reduction of mdr-1 expression after salvicine treatment in K562/A02 cells. In contrast, c-jun antisense oligodeoxy-nucleotides prevents salvicine-stimulated enhancement of c-Jun protein and reduction of mdr-1 gene expression[24]. Salvicine promotes phosphorylation of c-Jun-N-terminal kinase and c-Jun protein in MDR K562/A02 and parental K562 cells. Accordingly, salvicine enhances DNA binding activity of transcription factor activator protein 1 in the electrophoretic mobility shift assays. Moreover, c-Jun antisense oligodeoxynucleotides also inhibited salvicine-induced apoptosis and cytotoxicity in MDR and parental K562 cells[24]. A recent study found that salvicine induced equal ROS generation and glutathione depletion in both MDR and parental K562 cells (Cai Y, et al, unpublished data). Pretreatment of K562/A02 cells with NAC eliminated JNK phosphorylation, c-jun activation and P-gp downregulation induced by salvicine (Cai Y, et al, unpublished data). Together, salvicine-triggered oxidative stress stimulates c-Jun-N-terminal kinase phosphorylation and activation, resulting in c-Jun phosphorylation and activation. Activated c-Jun promotes expression of c-jun itself, represses mdr-1 transcription, and triggers pro-apoptotic signals, resulting in low mdr-1 expression and cell death (Figure 2).
Salvicine inhibits tumor metastasis activity related to Rho-dependent pathway and ROS-triggered p38MAPK pathway Except for its antiproliferative activity, salvicine has also been reported to possess antimetastatic effects[25]. Metastasis refers to the dissemination of cancer cells from initial tumor to distant sites and involves a series of processes, including loss of adhesion, acquisition of cell motility, extracellular proteolysis, and angiogenesis[26]. Salvicine significantly reduces the lung metastatic foci of MDA-MB-435 orthotopic xenograft, without obviously affecting primary tumor growth[25]. A comparison of gene expression profiles of primary tumors and lung metastatic focus between salvicine-treated and untreated groups using the CLOTECH Atlas Human Cancer 1.2 cDNA microarray revealed that genes involved in tumor metastasis, particularly those closely related to cell adhesion and motility, were obviously down-regulated, including fibronectin, integrin alpha3, integrin beta3, integrin beta5, FAK, paxillin, and RhoC[25]. Consis-tently, salvicine significantly downregulated RhoC at both mRNA and protein levels, greatly inhibited stress fiber formation and invasiveness of MDA-MB-435 cells, and markedly blocked translocation of both RhoA and RhoC from cytosol to membrane, indicating that the unique antimetas-tatic action of salvicine is closely related to Rho-dependent signaling pathway[25] (Figure 2). However, the mechanism that leads to downregulation of RhoC needs to be further clarified.
In an effort to explore the relationship between the antimetastatic activity of salvicine and ROS generation, salvicine was found to be capable of generating ROS in human breast cancer MDA-MB-435 cells that was significantly reversed by a ROS scavenger NAC(Zhou JC, et al, unpublished data). Salvicine also inhibits cell adhesion to fibronectin and collagen by disrupting the formation of both focal adhesions and actin stress fibers, leading to the rounding up of cells (Zhou JC, et al, unpublished data). In addition, salvicine downregulates β1 integrin affinity, clustering, and signaling via FAK and paxillin, and by contrast activated ERK and p38 MAPK (Zhou JC, et al, unpublished data). The specific inhibitor of MEK1/2 (U0126) or p38 MAPK (SB203580) abrogates the inhibitory effects of salvicine on β1 function and cell adhesion. Notably, NAC also abolishes the activation of ERK and p38 MAPK, thereby protecting β1 integrin affinity and restoring cell adhesion and spreading (Zhou JC, et al, unpublished data). Therefore, salvicine activates ERK and p38MAPK via triggering ROS generation, which drives inactivation of β1 integrin function and results in cell adhesion inhibition. Collectively, the antimetastatic activity of salvicine is related to the Rho-dependent signaling pathway and ROS-triggered p38MARK pathway (Figure 2). The relationship between these two pathways deserves further study.
Concluding remarks
Salvicine is a novel Topo II poison that binds to the ATPase domain of Topo II, promoting DNA-Topo II binding, inhibiting Topo II mediated DNA relegation and ATP hydrolysis. Salvicine displayed multi cellular effects including inducing Topo II inhibition, DNA damage, circumventing P-gp-mediated MDR and inhibiting tumor cell adhesion. All of these effects at least partially are dependent on ROS generation, indicating salvicine-elicited ROS plays a central role in the anticancer activity of salvicine (Figure 2). Thus, as an antitumor drug candidate, salvicine can also be used as a tool to study the complicated roles of ROS in different physiological processes in tumor cells, which will provide useful information for future clinical studies.
Acknowledgements
We are grateful to Dr Jin-sheng ZHANG for providing salvicine.
References
- Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol 2002;3:430-40.
- Giles GI, Sharma RP. Topoisomerase enzymes as therapeutic targets for cancer chemotherapy. Med Chem 2005;1:383-94.
- Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 2006;6:789-802.
- Qing C, Zhang JS, Ding J. In vitro cytotoxicity of salvicine, a novel diterpenoid quinone. Acta Pharmacol Sin 1999;20:297-302.
- Zhang JS, Ding J, Tang QM, Li M, Zhao M, Lu LJ, et al. Synthesis and antitumour activity of novel diterpenequinone salvicine and the analogs. Bioorg Med Chem Lett 1999;9:2731-6.
- Meng LH, Zhang JS, Ding J. Salvicine, a novel DNA topoisomerase II inhibitor, exerting its effects by trapping enzyme-DNA cleavage complexes. Biochem Pharmacol 2001;62:733-41.
- Meng LH, He XX, Zhang JS, Ding J. DNA topoisomerase II as the primary cellular target for salvicine in Saccharomyces cerevisiae. Acta Pharmacol Sin 2001;22:741-6.
- Hu CX, Zuo ZL, Xiong B, Ma JG, Geng MY, Lin LP, et al. Salvicine functions as novel topoisomerase II poison by binding to ATP pocket. Mol Pharmacol 2006;70:1593-601.
- Lu HR, Zhu H, Huang M, Chen Y, Cai YJ, Miao ZH, et al. Reactive oxygen species elicit apoptosis by concurrently disrupting topoisomerase II and DNA-dependent protein kinase. Mol Pharmacol 2005;68:983-94.
- Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry 2001;40:3316-23.
- Li TK, Chen AY, Yu C, Mao Y, Wang H, Liu LF. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev 1999;13:1553-60.
- Shiah SG, Chuang SE, Chau YP, Shen SC, Kuo ML. Activation of c-Jun NH2-terminal kinase and subsequent CPP32/Yama during topoisomerase inhibitor beta-lapachone-induced apoptosis through an oxidation-dependent pathway. Cancer Res 1999;59:391-8.
- Meng L, Ding J. Induction of bulk and c-myc P2 promoter-specific DNA damage by an anti-topoisomerase II agent salvicine is an early event leading to apoptosis in HL-60 cells. FEBS Lett 2001;501:59-64.
- Lu HR, Meng LH, Huang M, Zhu H, Miao ZH, Ding J. DNA damage, c-myc suppression and apoptosis induced by the novel topoisomerase II inhibitor, salvicine, in human breast cancer MCF-7 cells. Cancer Chemother Pharmacol 2005;55:286-94.
- Furuta T, Takemura H, Liao ZY, Aune GJ, Redon C, Sedelnikova OA, et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem 2003;278:20303-12.
- Jackson SK, Thomas MP, Smith S, Madhani M, Rogers SC, James PE. In vivo EPR spectroscopy: biomedical and potential diagnostic applications. Faraday Discuss 2004;126:103-17.
- Adachi N, Suzuki H, Iiizumi S, Koyama H. Hypersensitivity of nonhomologous DNA end-joining mutants to VP-16 and ICRF-193: implications for the repair of topoisomerase II-mediated DNA damage. J Biol Chem 2003;278:35897-902.
- Ding J, Miao ZH, Meng LH, Geng MY. Emerging cancer therapeutic opportunities target DNA-repair systems. Trends Pharmacol Sci 2006;27:338-44.
- Liu WJ, Jiang JF, Xiao D, Ding J. Down-regulation of telomerase activity via protein phosphatase 2A activation in salvicine-induced human leukemia HL-60 cell apoptosis. Biochem Pharmacol 2002;64:1677-87.
- Liu WJ, Zhang YW, Shen Y, Jiang JF, Miao ZH, Ding J. Telomerase inhibition is a specific early event in salvicine-treated human lung adenocarcinoma A549 cells. Biochem Biophys Res Commun 2004;323:660-7.
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005;19:2100-10.
- Qing C, Jiang C, Zhang JS, Ding J. Induction of apoptosis in human leukemia K-562 and gastric carcinoma SGC-7901 cells by salvicine, a novel anticancer compound. Anticancer Drugs 2001;12:51-6.
- Miao ZH, Tang T, Zhang YX, Zhang JS, Ding J. Cytotoxicity, apoptosis induction and downregulation of MDR-1 expression by the anti-topoisomerase II agent, salvicine, in multidrug-resistant tumor cells. Int J Cancer 2003;106:108-15.
- Miao ZH, Ding J. Transcription factor c-Jun activation represses mdr-1 gene expression Cancer Res 2003;63:4527-32.
- Lang JY, Chen H, Zhou J, Zhang YX, Zhang XW, Li MH, et al. Antimetastatic effect of salvicine on human breast cancer MDA-MB-435 orthotopic xenograft is closely related to Rho-dependent pathway. Clin Cancer Res 2005;11:3455-64.
- Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 2003;4:67-79.
- Berger JM, Gamblin SJ, Harrison SC, Wang JC. Structure and mechanism of DNA topoisomerase II. Nature 1996;379:225-32.
