Development of novel agents based on nitric oxide for the control of colon cancer1
Introduction
Colon cancer, the third leading cause of cancer in the United States and one of the most common human malignancies in the Western world, is highly preventable. It is estimated that there will be over 150 000 new cases and 52 000 deaths from colon cancer in 2007 in the United States[1]. These dreadful statistics underscore the pressing need for the development of new and effective methods for its prevention. Of the several strategies being currently pursued by various groups around the world, chemoprevention, defined as the application of natural or synthetic agents to prevent the development or recurrence of cancer, holds credible promise as an effective modality that will decrease the incidence of cancer in at-risk populations.
The last two decades have witnessed an almost explosive interest in screening both natural and synthetic compounds in the hopes of identifying suitable candidates for further development. An alternative approach has been to exploit knowledge from epidemiological studies and then proceed with the rational development of new agents and/or approaches. Nonsteroidal anti-inflammatory drugs and their prototype aspirin (ASA) represent a case in point. The ample documentation (epidemiological and recently interventional) of the chemopreventive effect of ASA has led to the exploration of the role of various NSAIDs in colon cancer chemoprevention. An almost natural extension of this work has been the recent effort to enhance the pharmacological properties of NSAIDs by combining them with a moiety that releases nitric oxide. This new class of compounds, the NO-donating NSAIDs (NO-NSAIDs), is the subject of the present review. After summarizing the salient points of the role of NO and also of conventional NSAIDs in cancer, we present our current understanding of the potential chemopreventive role of NO-NSAIDs against colon cancer.
Nitric oxide and its role in cancer
Nitric oxide (NO) is one of the simplest biological molecules in nature[2]. The discovery of its physiological role in the 1980s has had a major influence on many aspects of current biomedical research. Produced through the oxidation of L-arginine by the various nitric oxide synthases (NOS), NO is a free radical, a property that renders it very reactive and unstable. The 1998 Nobel Prize in Physiology or Medicine was awarded to R FURCHGOTT, L IGNARRO and F MURAD for their discoveries concerning NO as a signaling molecule in the cardiovascular system.
In addition to its role as an endogenous regulator of blood flow and thrombosis, NO is now accepted as a fundamental signaling molecule regulating virtually every critical cellular function, as well as a potent mediator of cellular damage in a wide range of conditions[3]. NO reacts readily with another radical, superoxide anion, forming peroxynitrite (this pair is considered to regulate the concentration of each other in a cell), which interacts with lipids, DNA, and proteins via direct oxidative reactions or via indirect, radical-mediated mechanisms. Chronic inflammatory diseases and cancer are among the many pathogenetic mechanisms mediated by peroxynitrite.
Although inflammation and DNA damage engendered by NO provide general links to cancer, many lines of evidence suggest the involvement of NO in discrete and sometimes critical steps of the complicated process that leads from normalcy to malignancy[4]. For example, NO affects tumor angiogenesis, metastasis, blood flow and immune surveillance[5]. Recent published reports indicate that even endothelial NO synthase (eNOS) can modulate cancer-related events, such as angiogenesis, apoptosis, cell cycle, invasion, and metastasis[6]. Furthermore, NO has the potential to enhance both radiotherapy and chemotherapy, but such strategies depend on achieving appropriate levels of NO[5].
NSAIDs and their role in colon cancer
Hippocrates, the Greek physician considered “the father of medicine,” wrote in the 5th century BCE that the willow bark improves aches, pains and fever. The modern era of NSAIDs began in 1829 with the isolation of salicin from the white willow, which was followed by the synthesis of aspirin in 1899 by Hoffman[7]. Currently, NSAIDs, comprising a chemically heterogeneous group of drugs, are being used as antipyretics, analgesics, and anti-inflammatory medications and in the prevention of myocardial infarction and stroke. NSAIDs are also used to close patent ductus arteriosus in neonates and in the treatment of dysmenorrhea. Lawrence Levine, demonstrating in 1973 that indomethacin reduced tumor size in fibrosarcoma-bearing mice[8], was the first to show the anticancer effect of NSAIDs. Soon after, several epidemiological studies showed that NSAIDs reduce both the risk of and mortality from colorectal cancer by about half[9]. Interventional studies conducted by Baron and his colleagues provided formal proof that aspirin does prevent colon cancer, albeit at a lower than expected rate[10,11].
Like all cancers, colon cancer is a congregation of abnormal cells and represents an imbalance between cell renewal and cell death. Following our original demonstration that NSAIDs reduce cell proliferation, induce apoptosis and change the distribution of cells in the cell cycle[12], many groups have attempted to decipher their molecular mechanism of action[13]. A surprising finding was that the effect of NSAIDs on cancer was independent of their inhibition of cyclooxygenase (COX), their best known pharmacological target[14]. This counterintuitive observation, initially received with skepticism, has been amply confirmed. Over 15 potential mechanisms have been described. Salient amongst them are inhibition of signaling via NF-κB, COX, NOS, PPAR (peroxisome-proliferator activated receptor), a family of nuclear hormone receptor and/or transcription factors, as well as inhibition of angiogenesis. All of these mechanisms converge directly or indirectly into a cell kinetic effect that eliminates preferentially neoplastic cells, thereby diminishing tumor cell mass. NSAIDs have their main effect in cancer prevention, being essentially ineffective in cancer treatment. This observation suggests that the fully developed cancer cells either have acquired resistance to NSAIDs or that the lower complexity of early neoplastic cells makes them vulnerable to NSAIDs.
There are two main pharmacological features of NSAIDs that essentially preclude their clinical application to colon cancer chemoprevention[15]. First, their efficacy is below 50% and, second, their safety profile is not favorable. It is important to recall that chemoprevention differs substantially from chemotherapy. In the latter, a medication, even one with significant side effects, is administered to treat patients with fully developed cancers that threaten their lives. Chemopreventive agents, in contrast, are given to healthy subjects for a cancer they may never develop. Thus the requirements for efficacy and safety are more stringent for chemopreventive agents. It is precisely these considerations that propel the search for alternatives to conventional NSAIDs as agents for cancer prevention. NO-NSAIDs are a promising development in this direction.
NO-donating NSAIDs as agents against colon cancer
NO-NSAIDs consist of a conventional NSAID to which the NO-releasing moiety –ONO2 has been attached via a chemical linker[16]. Representative members of this class of drugs are shown in Figure 1. The spacer can vary in its chemical structure, providing a great number of derivatives. NO-aspirin (NO-ASA) is at present the best-studied NO-NSAID. There are three positional isomers of the NO-ASA molecule, ortho, meta and para, generated by varying the position of the –CH2ONO2 group with respect to the ester bond linking the two benzenes[17].
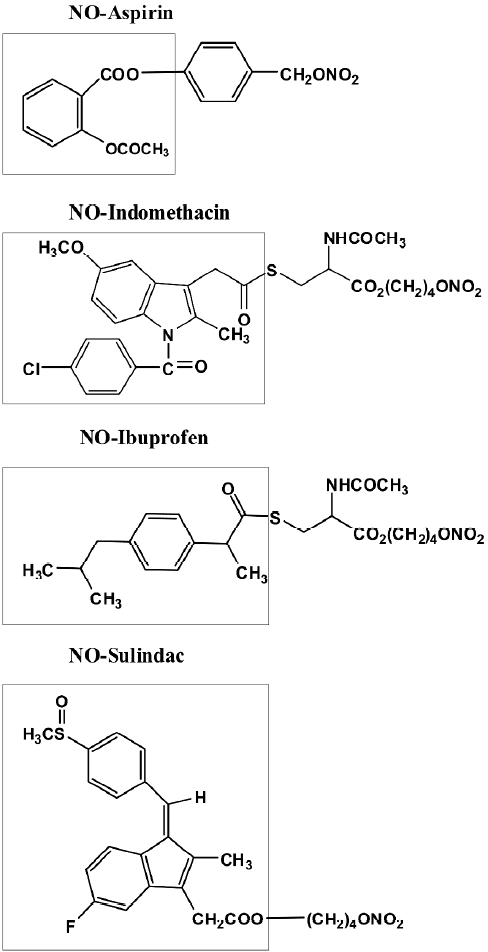
Williams et al were the first to publish data that showed that NO-ASA, NO-sulindac, and NO-ibuprofen have chemopreventive properties; all three compounds reduced the growth of cultured HT-29 human colon adenocarcinoma cells more potently than their corresponding NSAIDs[18]. This was achieved by inhibition of proliferation, induction of apoptosis and also inhibition of cell cycle phase transitions. Beyond classical apoptosis there was another form of cell death induced by NO-ASA that was named atypical cell death[17]. Atypical cell death, initially described in vitro, may actually occur in vivo. Ouyang et al recently described a sequence of morphological changes in intestinal tumors of Min mice treated with NO-ASA that strongly suggests its existence[19]. Gao et al also recorded the induction of apoptosis by NO-ASA in the SW480 colon cancer cell line and the induction of oxidative stress evidenced by enhanced levels of reactive oxygen species[20]. It was also observed that NO-ASA induced apoptosis in Min mice but did not do so in their wild type congenic controls.
Recent animal studies indicated that NO-ASA was more effective than its parental ASA in preventing colon cancer[21]. Studies on Min mice (they develop spontaneous intestinal tumors because of a truncating Apc mutation), rats treated with the carcinogen azoxymethane, and tumor xenografts, all generated similar results. In Min mice, 3 weeks of treatment with NO-ASA decreased the number of tumors by 55%[22]. Wallace and co-workers studied carcinogen-treated rats and used as endpoint aberrant crypt foci, which are the earliest premalignant lesions in the colon. NO-ASA reduced the number of aberrant crypt foci by 85% while ASA resulted in a 64% reduction[21]. Recently, Rao et al assessed the chemopreventive properties of NO-indomethacin (NCX 530) and meta NO-aspirin (NCX 4016) against azoxymethane-induced colon cancer in F344 rats[23]. NO-indomethacin and NO-ASA significantly suppressed both tumor incidence and multi-plicity. The degree of inhibition was more pronounced with NO-indomethacin than with NO-aspirin. Finally, the antitumor activity of para NO-ASA (NCX 4040) in combination with 5-fluorouracil (5-FU) or oxaliplatin was evaluated in vitro and in vivo in colon cancer models by Leonetti et al[24]. NO-ASA and 5-FU, combined in vitro, were always additive, regardless of the scheme used. Sequential NO-ASA and oxaliplatin treatment produced strong synergism in cell lines. In vivo this sequence caused higher reduction in tumor growth than single-drug treatments. The authors concluded that NO-ASA sensitizes colon cancer cell lines to the effect of antitumor drugs and that their combination could be useful for the clinical management of colon cancer.
A remarkably consistent finding in numerous animal studies, including those mentioned above, is the safety of NO-NSAIDs, which appears superior to that of their parent NSAIDs. Some clinical studies underscore the same, but they are very limited in scope[25,26].
The molecular mechanism underlying the effect of NO-NSAIDs remains unknown despite extensive work by us and others[27]. Significant effects have been noted on a variety of important signaling cascades including NF-κB, Wnt, mitogen activated protein kinase (MAPK), and NOS. On all of them the effect of NO-ASA, the agent studied in greatest detail, was inhibitory. Regarding the COX pathway, it was unexpectedly observed that NO-ASA induced the expression of COX-2[28]. When a comparison was made between the IC50s for cell growth inhibition and signaling inhibition in response to NO-ASA, the most important event was the inhibition of Wnt (β-catenin) signaling that occurred at concentrations way below those required for the inhibition of cell growth. In the study of Rao et al mentioned above[23], NO-indomethacin and NO-ASA inhibited the colon tumors’ COX activity and the levels of various prostaglandins, as well as NOS2 activity and β-catenin expression. In colonic crypts and tumors of animals fed these two NO-NSAIDs, cell proliferation was decreased.
Additional mechanistic studies have revealed modulation of drug metabolizing enzymes[29]. Such effects, leading to facilitated elimination of carcinogens, have been considered to represent a successful strategy for cancer chemo-prevention. NO-ASA induced the activity and expression of NAD(P)H:quinone oxireductase (NQO) and glutathione S-transferase (GST) both in vitro and in the liver of Min mice, Interestingly, NO-ASA induced the translocation of Nrf2 into the nucleus, an effect that paralleled the induction of NQO1 and GST P1-1. The induction of phase II enzymes by NO-ASA and the modulation of the Keap1-Nrf2 pathway were speculated to be part of NO-ASA’s mechanism of action against colon and other cancers.
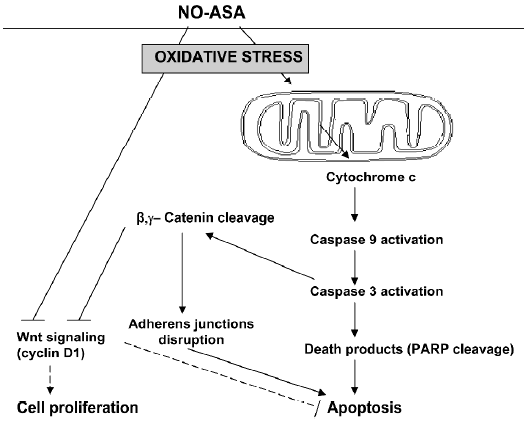
What appears to be the critical proximal event in the action of NO-ASA in cancer, however, is the induction of a state of oxidative stress in the target cell[20]. It is conceivable, that many of the changes in the signaling pathways mentioned above are derivative events, generated by redox changes that may either lead to reversible redox signaling or irreversible oxidative stress that culminates in cell death and thus elimination of the neoplastic cell. Figure 2 recapitulates the proposed mechanism of action of NO-ASA, highlighting changes concerning the (converging) apoptosis and Wnt signaling pathways.
Perspectives
NO-NSAIDs represent a promising recent development in cancer chemoprevention. The work accomplished to date by several laboratories exemplifies a rational and mechanism-based approach to cancer prevention. The NO-NSAID-based approach is the end result of a logical sequence starting with epidemiological observations on the effect of NSAIDs against colon cancer, mechanistic and formal interventional studies focusing mainly on conventional ASA and then the rather ingenious combination of NO and NSAIDs aiming to generate a superior agent.
The NO-based compounds have several promising features that generate reasonable expectations that they will prove useful chemopreventive agents. They include a sound pharmacological basis (NSAIDs plus NO); congruent preclinical evidence suggesting both efficacy and safety; and their presumed mechanism of action, which is based on ROS generation and includes a multi-pronged effect on several signaling pathways.
Only clinical testing will ascertain whether these compounds have any clinical value. Although the road to clinical application is long and the task arduous, several significant milestones have already been achieved, generating a level of excitement about these compounds.
Acknowledgments
We thank Stancy JOSEPH for critical comments and general help with the manuscript.
References
- Author X. Statistics for 2006. City of publication: American Cancer Society; 2006.
- Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol 2002;53:503-14.
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315-424.
- Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res 2004;555:107-19.
- Hirst D, Robson T. Targeting nitric oxide for cancer therapy. J Pharm Pharmacol 2007;59:3-13.
- Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res 2007;67:1407-10.
- Roberts J II, Morrow J. Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment gout. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001. p 687–31.
- Tashjian AH Jr, Voelkel EF, Goldhaber P, Levine L. Successful treatment of hypercalcemia by indomethacin in mice bearing a prostaglandin-producing fibrosarcoma. Prostaglandins 1973;3:515-24.
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst 2002;94:252-66.
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003;348:891-9.
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 2003;348:883-90.
- Shiff SJ, Qiao L, Tsai LL, Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest 1995;96:491-503.
- Shiff SJ, Rigas B. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal antiinflammatory drugs (NSAIDs) J Exp Med 1999;190:445-50. [comment].
- Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol 1996;52:237-45.
- Rayyan Y, Williams J, Rigas B. The role of NSAIDs in the prevention of colon cancer. Cancer Invest 2002;20:1002-11.
- del Soldato P, Sorrentino R, Pinto A. NO-aspirins: a class of new anti-inflammatory and antithrombotic agents. Trends Pharmacol Sci 20:319-23.
- Kashfi K, Borgo S, Williams JL, Chen J, Gao J, Glekas A, et al. Positional isomerism markedly affects the growth inhibition of colon cancer cells by nitric oxide-donating aspirin in vitro and in vivo. J Pharmacol Exp Ther 2005;312:978-88.
- Williams JL, Borgo S, Hasan I, Castillo E, Traganos F, Rigas B. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: implications for colon cancer chemoprevention. Cancer Res 2001;61:3285-9.
- Ouyang N, Williams JL, Rigas B. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR){delta} expression in APCmin/+ mice proportionally to their tumor inhibitory effect: Implications for the role of PPAR{delta} in carcinogenesis. Carcinogenesis 2006;27:232-9.
- Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci USA 2005;102:17207-12.
- Bak AW, McKnight W, Li P, Del Soldato P, Calignano A, Cirino G, et al. Cyclooxygenase-independent chemoprevention with an aspirin derivative in a rat model of colonic adenocarcinoma. Life Sci 1998;62:L367-73.
- Williams JL, Kashfi K, Ouyang N, del Soldato P, Kopelovich L, Rigas B. NO-donating aspirin inhibits intestinal carcinogenesis in Min (APC(Min/+)) mice. Biochem Biophys Res Commun 2004;313:784-8.
- Rao CV, Reddy BS, Steele VE, Wang CX, Liu X, Ouyang N, et al. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: effects on molecular targets. Mol Cancer Ther 2006;5:1530-8.
- Leonetti C, Scarsella M, Zupi G, Zoli W, Amadori D, Medri L. Efficacy of a nitric oxide-releasing nonsteroidal anti-inflammatory drug and cytotoxic drugs in human colon cancer cell lines in vitro and xenografts. Mol Cancer Ther 2006;5:919-26.
- Fiorucci S, Santucci L, Gresele P, Faccino RM, Del Soldato P, Morelli A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology 2003;124:600-7.
- Iconomou G, Kalofonos HP, Koutras AK, Vagenakis AG, Rigas B. Pilot study of nitric oxide-donating aspirin in patients with pancreatic cancer pain. J Support Oncol 2006;4:168.
- Rigas B, Kashfi K. Nitric-oxide-donating NSAIDs as agents for cancer prevention. Trends Mol Med 2004;10:324-30.
- Williams JL, Nath N, Chen J, Hundley TR, Gao J, Kopelovich L, et al. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction and beta-catenin/T-cell factor signaling, nuclear factor-kappaB, and NO synthase 2 inhibition: implications for chemoprevention. Cancer Res 2003;63:7613-8.
- Gao J, Kashfi K, Liu X, Rigas B. NO-donating aspirin induces phase II enzymes in vitro and in vivo. Carcinogenesis 2006;27:803-10.
