Prevention and therapy of squamous cell carcinoma of the rodent esophagus using freeze-dried black raspberries1
Introduction
Squamous cell carcinoma (SCC) accounts for >90% of esophageal cancers worldwide[1]. Esophageal SCC develops through a progressive sequence from mild to severe dysplasia, carcinoma in situ and, finally, invasive carcinoma[2,3]. This cancer shows marked variation in geographical distribution, with high frequencies in parts of China, Iran, South Africa, Uruguay, France, Italy and Puerto Rico[4]. The highest incidences are in the Shansi, Henan and Hopei provinces in China, where the age-adjusted mortality rates are 151/100 000 for men and 115/100 000 for women[5]. Risk factors for the disease include tobacco smoking, alcohol consumption, ingestion of salt-pickled, salt-cured and moldy foods, deficiencies in certain dietary vitamins and minerals and, potentially, infection with human papilloma virus (HPV)[4]. Research in China provides evidence that N-nitroso compounds and their precursors are etiological factors for esophageal SCC in the high-incidence areas[6,7]. Nitrosamine carcinogens such as N-nitrosomethylbenzylamine (NMBA) and N-nitrosomethylamylamine (NMAA) have been identified in the diets and gastric juices collected from subjects in China[7]. In addition, contaminated foods often contain nitrates, nitrites and secondary and tertiary amines, which act as precursors for the formation of nitrosamine carcinogens in the acidic conditions of the stomach[8]. Thus, lifestyle changes, especially the avoidance of tobacco and alcohol use and the elimination of high salt and moldy foods, might be expected to reduce the incidence and mortality of this disease. Chemo-prevention may be another feasible approach and may have special relevance in high-incidence areas of the world[4].
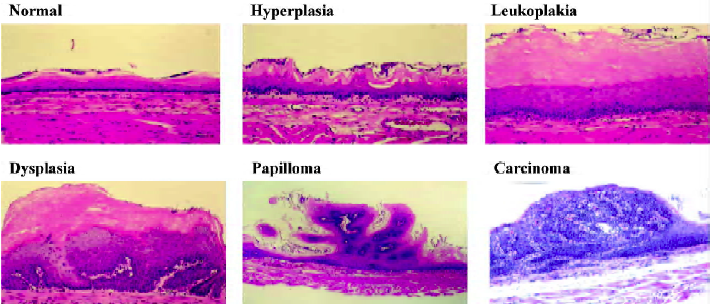
Nitrosamine-induced tumorigenesis in the Fischer-344 rat has proven to be a valuable animal model for studies of the molecular biology and chemoprevention of esophageal SCC[4]. Several nitrosamines, including NMBA and the tobacco-specific nitrosamine, N-nitrosonornicotine (NNN), induce tumorigenesis in the rat esophagus[8]. NMBA is by far the most potent inducer of tumors in the rat esophagus. Its metabolic activation to DNA damaging species in the esophagus, especially the formation of O6-methylguanine (O6-MeGua) adducts in esophageal DNA, has previously been described[4]. Repeat subcutaneous injection of NMBA in F-344 rats results in esophageal tumor formation within 15–26 weeks (Figure 1). Several preneoplastic lesions produced in NMBA-treated rat esophagus closely mimic lesions observed in the human esophagus. These lesions include simple hyperplasia, leukoplakia and epithelial dysplasia (Figure 2). Squamous papilloma is the predominant tumor type seen in the rat esophagus model. The incidence of SCC is rather low because the rats often succumb to the occlusive effects of large papillomas in their esophagi before carcinomas can develop. In a typical tumor bioassay, subcutaneous injections of NMBA at either 0.25 or 0.5 mg/kg body weight three times per week for 5 weeks, or once per week for 15 weeks, result in a 100% tumor incidence by 26 weeks[9,10]. On average, these two concentrations of NMBA will produce from 2–4 or 4–8 tumors per esophagus, respectively, at 26 weeks. In the past several years our laboratory and others have used this model to develop surrogate end-point biomarkers, identify novel targets for intervention and therapy and evaluate putative chemoprevention agents against esophageal SCC[11].

Since the early 1990s, our laboratory has taken a “food-based” approach to the prevention of gastrointestinal tract cancers using freeze-dried berries[12,13]. The rationale for the choice of berries and, in particular, black raspberries for cancer prevention has been previously described[13]. Black raspberries contain multiple agents that, by themselves, exhibit chemopreventive effects in animals, including vitamins A, C, E, and folic acid, calcium, selenium, β-sitosterol, ellagic and ferulic acids, quercetin, and at least 5 anthocyanins[10,12]. The high level of anthocyanins is responsible for the dark color and high-antioxidant potential of black raspberries[13]. In an initial study, freeze-dried black raspberries (FBR), administered at specific concentrations in a synthetic diet, produced significant decreases in NMBA-induced esophageal tumors in rats[10]. Specifically, 5% and 10% dietary FBR produced a 39% and 49% reduction, respectively, in esophageal tumor multiplicity when administered in the diet 2 weeks before, during and after treatment of rats with NMBA (see Figure 3A for the complete carcinogenesis protocol). The berries were found to reduce O6-MeGua adduct formation, preneoplastic lesion development and cell proliferation rates in NMBA-treated esophagus. In the same study, 5% and 10% FBR produced 37% and 31% reductions in tumor multiplicity, respectively, when administered in the diet immediately after cessation of NMBA treatment and until the end of the bioassay (see Figure 3B for the anti-promotion/progression protocol). Using the anti-promotion/progression protocol, Chen et al[14,15] have shown that 5% dietary FBR downregul-ates the mRNA and protein expression levels of cyclooxy-genase-2 (COX-2), inducible nitric oxide synthase (iNOS), c-Jun (a component of activator protein-1 (AP-1), and vascular endothelial growth factor (VEGF) in NMBA-treated esophagus, and these reductions correlated with lower levels of prostaglandin E2 (PGE2), nitrate/nitrite and microvessel density in NMBA-treated esophagus, respectively. FBR, therefore, can reduce the expression levels and activities of genes associated with cellular proliferation, inflammation and angiogenesis in the rat esophagus.
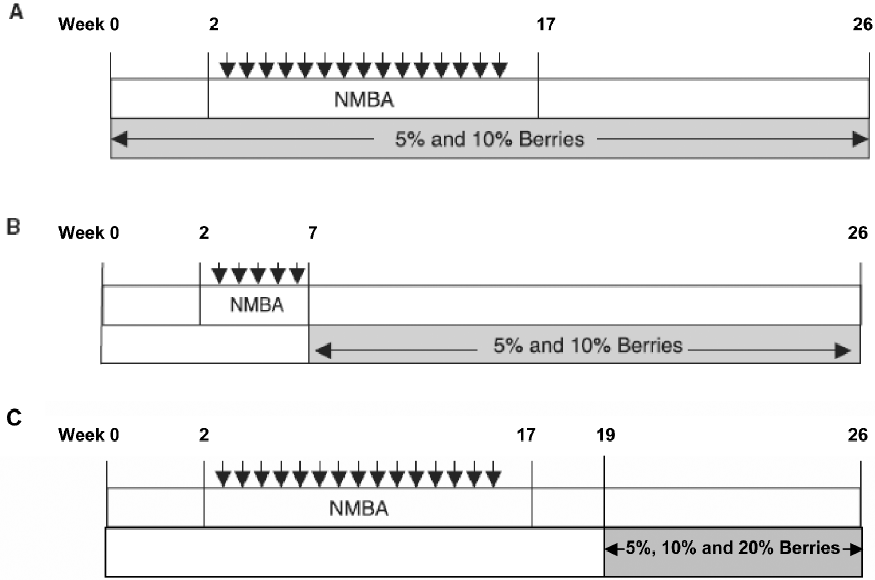
In contrast to previous studies that have examined black raspberries for their anti-initiation and anti-promotion/progression effects in the rat esophagus, the present study evaluated FBR for their potential therapeutic effects on esophageal carcinogenesis (see Figure 3C for the therapeutic protocol). Specifically, 5%, 10% and 20% dietary FBR were tested for their ability to reduce the incidence, number and size of fully developed papillomas in NMBA-treated esophagus. This study is important in that there is considerable interest as to whether or not chemopreventive agents, including natural foods, can be used in conjunction with standard therapies (ie surgery, radiotherapy and chemo-therapy) for the treatment of esophageal SCC and/or for the prevention of its recurrence following treatment.
Materials and methods
Animals Male F-344 rats, 4 weeks of age, were purchased from Harlan-Sprague Dawley (Indianapolis, IN, USA). The animals were housed under standard conditions (20±2 ºC; 50%±10% relative humidity; 12-h light/dark cycles) and maintained on a modified AIN-76A diet as previously described[10]. Food and water were provided ad libitum and hygienic conditions were maintained by twice weekly cage changes. Body weight and food consumption measurements were recorded biweekly after administration of the berry diet and for the duration of the study.
Chemicals NMBA, obtained from Ash Stevens (Detroit, MI, USA), was greater than 98% pure as determined by high performance liquid chromatography. Dimethyl sulfoxide (DMSO) was purchased from the Sigma Chemical Company (St Louis, MO, USA).
Diet preparation Black raspberries (Rubus occidentalis) of the Bristol variety were supplied by the Stokes Raspberry Farm (Wilmington, OH, USA). The berries were picked, washed with water and frozen on the farm at -20 ºC within 2–4 h of the time of picking[12]. The berries were then shipped frozen to Van Drunen Farms (Momence, IL, USA) where they were freeze-dried. The FBR were packaged in double polyethylene bags, placed in carton boxes and stored at -20 ºC. The berries were then shipped frozen to the Parker Food Science and Technology Building, Ohio State University, and stored frozen until they were used in the experiments. Berries were analyzed routinely for content of certain vitamins, minerals, phenols, carotenoids and phytosterols by Covance Laboratories (Madison, WI, USA) and for anthocyanin and ellagic acid content in the laboratory of Dr Steven Schwartz, Department of Food Science and Techno-logy, Ohio State University. Data from these analyses indicate that, with the exception of vitamin C, which degrades in frozen berries, the components measured in FBR remain relatively stable for at least 2 years when the berries are stored at -20 °C. On a biweekly basis, FBR were mixed for 20 min in a modified AIN-76A diet at concentrations of 5%, 10% and 20% using a Hobart mixer. The cornstarch in the diet was reduced by 5%, 10%, and 20%, respectively, to provide a similar caloric intake in the diets.
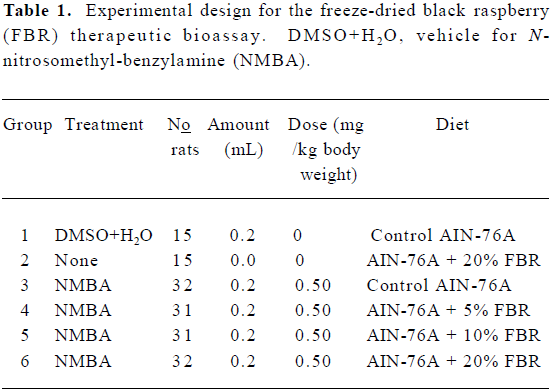
Animal bioassay Rats were randomized into 6 groups and placed on a AIN-76A diet (Table 1). They were then treated as follows: Group 1 was injected subcutaneously with 0.2 mL of a solution containing 20% DMSO in water (the solvent for NMBA) once per week for 15 weeks. Group 2 was fed a diet containing 20% FBR only. Groups 3–6 were injected subcutaneously with NMBA (0.5 mg/kg body weight) once per week for 15 weeks. To monitor for papilloma development, at the beginning of week 19, 5 rats from the NMBA control group (Group 3) were killed and their tumors were counted. The animals had an average of 5–6 papillomas per esophagus, all of which exceeded 1 mm in diameter. Immediately, therefore, animals in Groups 4–6 were placed on diets containing 5%, 10% and 20% FBR, respectively (Figure 3C). The rats remained on the berry diets until 26 weeks when animals in all groups were killed. At necropsy, the esophagus of each animal was opened longitudinally and the surface tumors were mapped, counted and measured. Lesions greater than 0.5 mm in diameter were considered to be tumors. Assuming a spheroid shape, a volume estimate for each papilloma was calculated[16]. Each esophagus was fixed on laminated cards in 10% neutral buffered formalin (NBF) to be processed for histopathology. In addition, sections of the liver, colon, kidney and spleen were harvested and fixed in NBF to evaluate possible toxicity of FBR.
Full table
Immunohistochemical analysis of cell proliferation At the end of the study, the entire esophagus from 5 rats per group was stained for the cell proliferation marker, proliferating cell nuclear antigen (PCNA), as previously described[10]. In brief, following antigen retrieval and treatment with 3% H2O2, tissue sections were incubated with the primary antibody, monoclonal mouse anti-PCNA (BioGenex, San Ramon, CA, USA) for 30 min. Rat-absorbed link secondary antibody (biotinylated anti-immunogobulin) followed for 30 min, and streptavidin-horseradish peroxidase for another 30 min. Slides were then incubated in 3,3'-diaminobenzidene for 3.5 min to permit biomarker visualization. The slides were counterstained with hematoxylin, dehydrated and cover slipped with Permount (Fisher Scientific, Pittsburgh, PA, USA). Positive (colon) and negative controls (mouse antiserum) were included in each run. PCNA-stained slides were viewed at 200× with a Nikon bright-field microscope mounted with a high-resolution spot camera. The camera was interfaced with a computer containing a matrix frame grabber board and image analysis software (Simple PCI Imaging Systems; Complix, Cranberry Township, PA, USA). The basal layer of the esophagus was scanned and a minimum of 10 fields (1500–2000 cells) was quantified to determine the mean labeling index (LI). The LI was computed by dividing the positive nuclear-stained area by the total nuclear area; the LI was expressed as a percentage.
Statistical analysis All statistical procedures were carried out using the NCSS 97 statistical software package (NCSS Statistical Software, Kaysville, UT, USA). Body weight, food consumption, tumor multiplicity and tumor size data were analyzed for statistical significance (P<0.05) using
For survival analysis, the means, medians and standard deviations of tumor numbers and average volumes were computed for each group defined by treatment (Vehicle, 20% BRB, NMBA, NMBA + 5% BRB, NMBA + 10% BRB, NMBA +20% BRB). The groups were compared using a one-way
Results
There were no differences in mean body weight or food consumption for rats in all groups prior to being fed berry diets. From weeks 23 to 26, however, there were significant reductions in body weight in rats treated with NMBA only or with NMBA and all three dietary concentrations of berries compared with Group 1 and 2 controls (data not shown). These reductions in body weight resulted from increases in the number and size of tumors throughout the esophagus, leading to a reduced passage of food.
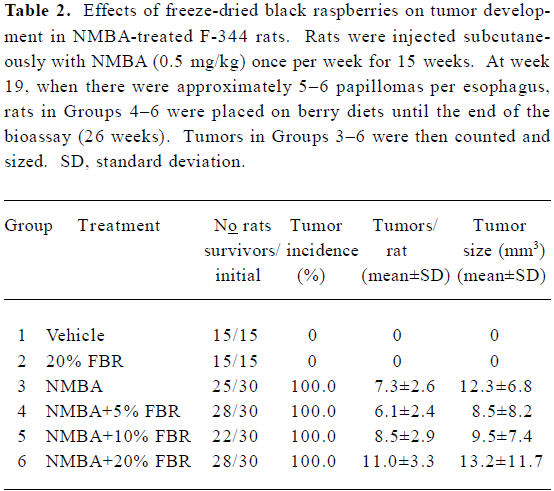
There were no significant differences in esophageal tumor incidences, numbers or volumes in NMBA-treated rats fed 5%, 10% or 20% FBR (Groups 4–6) compared with rats treated with NMBA only (Group 3) (Table 2). Histopathological examination of sections of the liver, colon, stomach, kidney and spleen from animals fed 20% FBR indicated that the berries did not produce toxic effects in any of these organs.
Full table
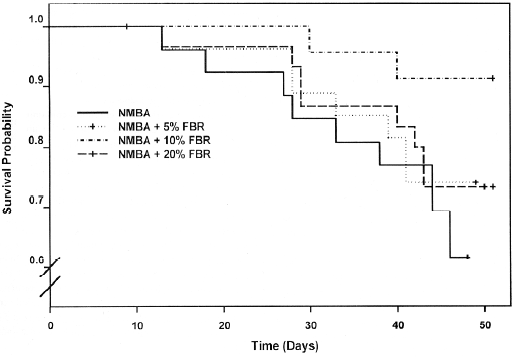
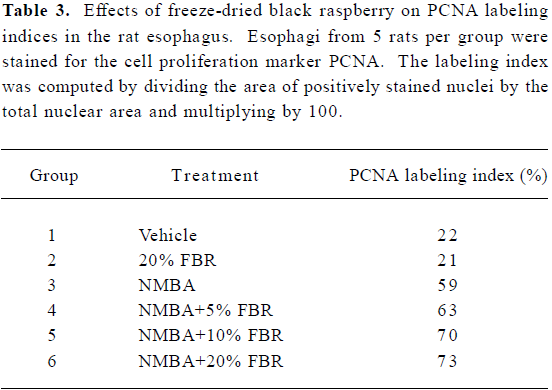
A Kaplan-Meier survival curve of animals in Groups 3–6 (NMBA-treated rats with and without berry diets) is shown in Figure 4. Included in the survival data were rats that died during the course of the experiment from week 19 through to week 26, as well as those killed at the end of the bioassay (week 26). Overall, there were no significant differences in survival between rats in Groups 3–6 (P=0.146). The effects of FBR on the PCNA LI in the esophagus of rats in all groups are shown in Table 3. As indicated, the PCNA LI in the esophagus of rats fed 20% FBR (Group 2) was similar to that in vehicle controls (Group 1). NMBA treatment (Group 3) resulted in a >2-fold increase in the PCNA LI relative to vehicle or berry controls (Groups 1 and 2). The esophagi of NMBA-treated animals fed 5%, 10% or 20% (Groups 4–6) had somewhat higher PCNA LI than the NMBA controls (Group 3); however, these differences were not significant.
Full table
Discussion
We have reported on the ability of FBR to prevent the development of NMBA-induced papillomas in the rat esophagus when the berries were fed at 5% and 10% of the diet either before, during or after treatment of rats with the carcinogen (Figure 3A) or shortly after carcinogen treatment until the end of the bioassay (Figure 3B)[10,12]. When administered in the diet before, during and after treatment of the rats with NMBA, the berries were shown to influence the metabolism of NMBA leading to a reduced formation of O6-MeGua adducts in esophageal DNA[10]. Subsequently, treatment of rats with dietary FBR was shown to inhibit the metabolism of NMBA in esophageal explant cultures and in liver microsomes in vitro, and to stimulate the activity of glutathione-S-transferase in the liver, all of which could be responsible for the reduced rate of adduct formation in vivo[18]. In this same protocol (Figure 3A), dietary berries were found to reduce the growth rate of preneoplastic cells in NMBA-treated esophagi almost to the level seen in untreated esophagi[10]. Subsequently, FBR were shown to downregul-ate the mRNA and protein expression levels of COX-2, iNOS, c-Jun and VEGF in pre-neoplastic esophagus when administered in the diet shortly after treatment of the rats with NMBA (Figure 3B)[14,15]. These changes in gene expression were associated with reduced levels of PGE2, nitrate/nitrite and microvessel density in pre-neoplastic esophageal epithelium, indicating that berries influence the expression of genes involved in cell proliferation, inflammation and angiogenesis. In vitro studies involving the induction of luciferase reporter genes with benzo(a)pyrene-7,8-diol-9,10-epoxide (B[a]PDE) in mouse epidermal JB-6 clone 41 cells have shown that extracts of FBR inhibit signal transduction pathways leading to activation of AP-1 and NF-κB, resulting in downregulation of VEGF and COX-2 expression[19–21]. These extracts were also shown to selectively inhibit the proliferation of oral cavity cancer cells in vitro and to stimulate their apoptosis[22].
In the present study, FBR were evaluated for their potential therapeutic effects against NBMA-induced rat esophageal papillomas. FBR were added to the diet at week 19 of the standard bioassay, when the esophagi contained an average of 5–6 papillomas each (Figure 3C). The results of this study were disappointing in that the berries did not reduce the incidence, number or size of papillomas when given in the diet for a period of 7 weeks. In addition, although there was a trend toward enhanced survival in NMBA-treated animals fed berries compared with NMBA controls, the results were not significant. The reasons for the lack of response of already developed papillomas to berry treatment are unknown, however, it is possible that the active compounds in berries, some of which appear to be the anthocyanins[23], are not well absorbed into the papillomas. This might be because of the relatively thick layer of kertain on the papilloma surface. It is also possible that the conversion of dysplastic lesions to papillomas is accompanied by genetic events that render the papillomas resistant to the inhibitory effects of berry compounds. Finally, the treatment period (7 weeks) may have been too short for the FBR to be effective. Unfortunately, the treatment period could not be extended because of the progressive loss of NMBA-treated animals, beginning at 22 weeks of the bioassay. This loss resulted from the inability of rats with developed papillomas to swallow food and to respiratory failure in rats with large papillomas that press against the trachea and restrict the passage of air.
Acknowledgements
We thank Dr Amy FERKETICH and Dr Li-shu WANG for statistical analysis of the data, and Mr Ronald NINES for assistance with the animal bioassay.
References
- Souza RF. Molecular and biologic basis of upper gastrointestinal malignancy-esophageal carcinoma. Surg Oncol Clin N Am 2002;11:257-72.
- Anani PA, Gardiol D, Savary M, Monnier P. An extensive morphological and comparative study of clinically early and obvious squamous cell carcinoma of the esophagus. Pathol Res Pract 1991;187:214-9.
- Kuwano H, Watanabe M, Sadanaga N, Ikebe M, Mori M, Sugimachi K. Squamous epithelial dysplasia associated with squamous cell carcinoma of the esophagus. Cancer Lett 1993;72:141-7.
- Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis 2001;22:1737-46.
- Munoz N, Buiatti E. Chemoprevention of oesophageal cancer. IARC Sci Publ 1996;136:27-33.
- Li MH, Ji C, Cheng SJ. Occurrence of nitroso compounds in fungi-contaminated foods: a review. Nutr Cancer 1986;8:63-9.
- Lu SH, Chui SX, Yang WX, Hu XN, Guo LP, Li FM. Relevance of N-nitrosamines to esophageal cancer in China. In: O’Neill IK, Chen J, Bartsch H, editors, Relevance to human cancer of N-nitroso compounds, tobacco smoke and mycotoxin; v 105. Lyon: IARC Scientific Publications; 1991. p 11–7.
- Hecht SS, Stoner GD. Lung and esophageal carcinogenesis. In: Aisner J, Arriagada R, Green MR, Martini N, Perry MC, editors, Comprehensive textbook of thoracic oncology. Baltimore: Williams and Wilkins; 1996. p 25–50.
- Siglin JC, Barch DH, Stoner GD. Effects of dietary phenethyl isothiocyanate, ellagic acid, sulindac and calcium on the induction and progression of N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Carcinogenesis 1995;16:1101-6.
- Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res 2001;61:6112-9.
- Stoner GD, Wang LS, Chen T. Chemoprevention of esophageal squamous cell carcinoma. Toxicol Appl Pharmacol 2007. [Epub ahead of print].
- Stoner GD, Chen T, Kresty LA, Aziz RM, Reinemann T, Nines R. Protection against esophageal cancer in rodents with lyophilized berries: potential mechanisms. Nutr Cancer 2006;54:33-46.
- Stoner GD, Wang L-S, Zikri N, Chen T, Hecht SS, Huang C, et al. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol 2007. [Epub ahead of print].
- Chen T, Hwang H, Rose ME, Nines RG, Stoner GD. Chemopreven-tive properties of black raspberries in N-nitrosomethylbenzyl-amine-induced rat esophageal tumorigenesis: down-regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-Jun. Cancer Res 2006;66:2853-9.
- Chen T, Rose ME, Hwang H, Nines RG, Stoner GD. Black raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis 2006;27:2301-7.
- Harris GK, Gupta A, Nines RG, Kresty LA, Habib SG, Frankel WL, et al. Effects of lyophilized black raspberries on azoxy-methane-induced colon cancer and 8-hydroxy-2'-deoxyguanosine levels in the Fischer 344 rat. Nutr Cancer 2001;40:125-33.
- Klein J, Moeschberger M, editors. Survival analysis: techniques for censored and truncated data. New York: Springer Publishers; 2003.
- Reen RK, Nines R, Stoner GD. Modulation of N-nitrosomethyl-benzylamine metabolism by black raspberries in the esophagus and liver of Fischer 344 rats. Nutr Cancer 2006;54:47-57.
- Huang C, Huang Y, Li J, Hu W, Aziz R, Tang MS, et al. Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor kappa B by black raspberry extracts. Cancer Res 2002;62:6857-63.
- Huang C, Li J, Song L, Zhang D, Tong Q, Ding M, et al. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/Akt pathway. Cancer Res 2006;66:581-7.
- Lu H, Li H, Zhang D, Stoner GD, Huang C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: From transcription factors to their target genes. Nutr Cancer 2006;54:69-78.
- Rodrigo K, Rawal Y, Renner R, Schwartz S, Tian Q, Larsen P, et al. Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutr Cancer 2006;54:58-68.
- Hecht SS, Huang C, Stoner GD, Li J, Kenney PM, Sturla SJ, et al. Identification of cyanidin glycosides as constituents of freeze-dried black raspberries which inhibit anti-benzo[a]pyrene-7,8-diol-9,10-epoxide-induced NF-kappaB and AP-1 activity. Carcinogenesis 2006;27:1617-26.
