Oriental herbs as a source of novel anti-androgen and prostate cancer chemopreventive agents1
Introduction
Oriental herbal medicine has been used since ancient times to treat malignancies in China and other Asian countries. Although some scientific evidence exists regarding a few herbal therapies, for most herbal remedies and formulas the key questions for Western medical providers are quality control/assurance issues for the herbal products, their safety and efficacy and adverse interactions with other medications. Systematic characterization of active phytochemicals in medicinal herbs and their mechanisms of action are important for providing the rationale for their efficacy and for transforming herbal practices into evidence-based medicine. This review focuses on our discovery of decursin and its naturally occurring pyranocoumarin analogs from an Oriental herbal formula containing Korean Angelica gigas Nakai (Dang Gui) root as a novel class of anti-androgen and androgen receptor (AR) signaling agents with excellent potential for prostate cancer (PCa) chemoprevention.
Androgen receptor and signaling as an impor-tant target for prostate cancer chemopre-vention
Androgen and AR-mediated signaling are crucial for the development and function of the normal prostate as well as for PCa[1,2]. The importance of androgen in PCa is supported by the observations that PCa rarely occurs in eunuchs or in men with a deficiency in 5α-reductases, the enzymes that convert testosterone to its active metabolite 5α-dihydrotes-testerone (DHT)[1]. Androgen/hormonal-ablation therapies are standard treatments for invasive metastatic PCa because a large percentage of the cancer cells are still responsive to androgen. In hormone-refractory cancer that inevitably arises after hormonal ablation therapy, which almost always brings about 3–5 years of remission, most of the PCa cells still retain wild-type AR. The AR is a ligand-dependent transcrip-tion factor, mediating the genomic effects of androgen action in the prostate and PCa cells. Novel approaches using an anti-AR ribozyme and inactivating monoclonal antibodies to reduce AR protein/function[3,4] and a decoy oligonucleotide containing an androgen-responsive element to sequester endogenous AR[5] have been developed. The data support the critical role of AR in PCa cell survival and proliferation in even the androgen-independent stage. More recent work using siRNA to knockdown AR further confirms these findings[6]. Therefore, agents that suppress AR abundance and ligand-dependent and ligand-independent signaling will be especially attractive for chemoprevention and therapy of PCa.
A clinical trial with finasteride (Proscar), which inhibits 5α-reductase II within the prostate gland, has shown a significant reduction in total PCa incidence[7]. However, PCa that developed in subjects in the intervention group appeared to be more advanced in tumor stages than that in the placebo group, raising doubts about the overall survival benefit of this single-target approach. Novel agents that target multiple aspects of androgen and AR signaling will be more desirable.
Screening biomarkers for activity-guidedfrac-tionation of novel anti-AR agents
With respect to biomarkers of androgen and AR signaling, prostate specific antigen (PSA) is a gene tightly regulated by androgen in normal prostate and some PCa cells[8]. A member of the kallikrein family (KLN3), PSA is a serine protease with highly prostate-specific expression and is elevated in the blood circulation of patients with PCa. Circulating PSA is widely used clinically as a marker for PCa screening and is particularly useful as an indicator of PCa response to therapy and recurrence[8]. The LNCaP human PCa cells are perhaps the best studied in vitro model for androgen and AR signaling in PCa. They possess a high-affinity mutant AR and produce high levels of PSA, which is extremely responsive to androgen stimulation[9,10]. In addition to PSA expression, another known outcome of androgen deprivation or blockage of AR signaling in these cells is the induction of neurite-like projections that have been termed “neuroendocrine differentiation” (NED)[11,12]. Androgen signaling represses these morphological manifestations and the associated molecular markers such as neuron-specific enolase[11]. Therefore, we chose this cell line as the primary cell target for screening novel anti-androgen and AR agents using PSA and NED as key functional biomarkers.
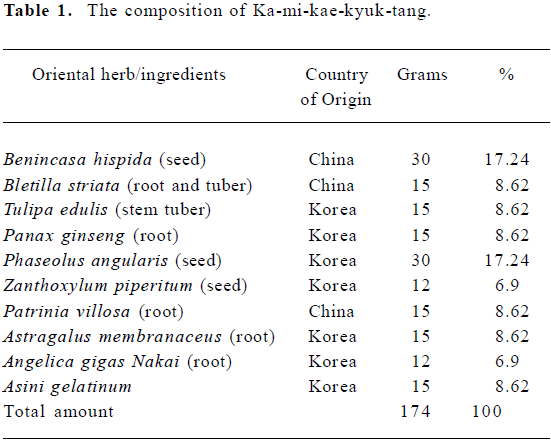
Our starting material was the ethanol extract of a traditional formula, Ka-mi-kae-kyuk-tang (KMKKT; see composition in Table 1). We have shown recently that it possesses broad-spectra in vivo anti-cancer activities of targeting angiogenesis, apoptosis and metastasis without any adverse effect on body weight[13].
Full table
Findings with KMKKT and AGN extracts in terms of PSA suppression and growth inhibition activities[14]
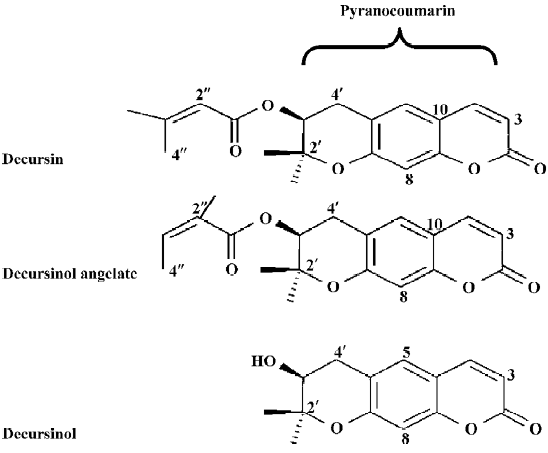
Using the LNCaP cell model, we found that KMKKT ethanol extract suppressed the expression of PSA mRNA and protein (IC50 ~7 µg/mL, 48 h exposure), inhibited androgen-induced cell proliferation and blocked the ability of androgen to suppress NED at exposure concentrations that caused G1 arrest, but far below doses that cause apoptosis. Mechanistically, KMKKT extract inhibited androgen-stimulated AR translocation to the nucleus and downregulated AR protein abundance without affecting the AR mRNA level. To identify the herb(s) containing anti-androgen activities, we prepared an ethanol extract of each of the 10 herbs. The AGN extract exerted a concentration-dependent suppression of cellular PSA and the IC50 for AGN extract was estimated to be ~1 µg/mL[14]. As decursin is a known major component of Korean AGN[15–17], which we confirmed using TLC and HPLC analyses[14], we focused on this phytochemical (structure shown in Figure 1) as a likely candidate.
Decursin decreases PSA expression and AR protein abundance[14]
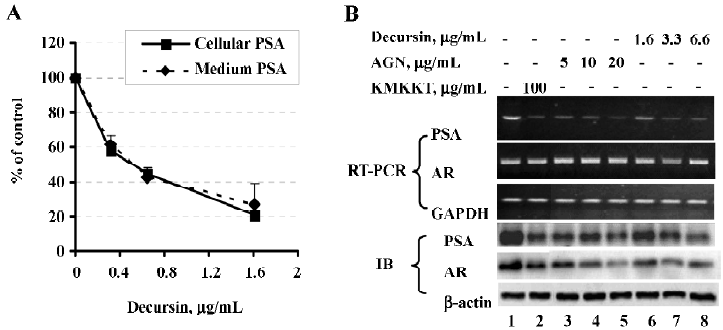
Exposure of LNCaP cells to purified decursin for 48 h in complete medium decreased cellular and secreted PSA with an IC50 of ~0.4 µg/mL (1.3 µmol/L) (Figure 2A). Exposure for 24 h to decursin (1.6, 3.3, and 6.6 µg/mL, corresponding to 5, 10 and 20 µmol/L) and AGN extract (5, 10, and 20 µg/mL) decreased, in a concentration-dependent manner, PSA mRNA and protein abundance (Figure 2B), recapitulating the effects of KMKKT. Like KMKKT, decursin or AGN extract did not affect the AR mRNA level detected by RT-PCR (Figure 2B), but did decrease the AR protein abundance detected by immunoblot (IB) (Figure 2B).
Growth suppression through G1 arrest[14]
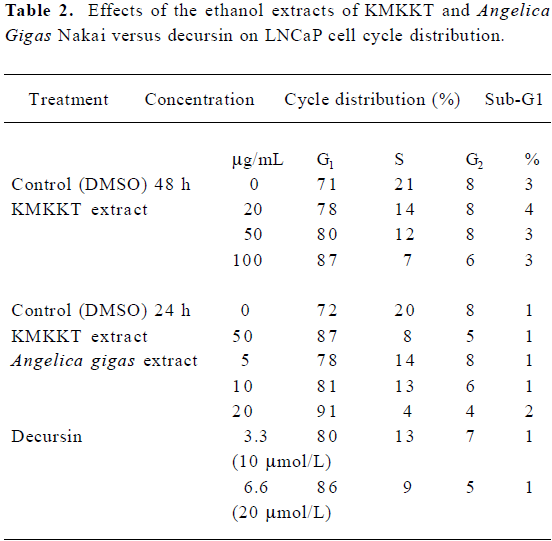
Exponentially growing LNCaP cells were treated in complete medium containing 10% whole serum for 24 or 48 h before flow cytometry analyses. AGN extract and decursin induced potent G1 arrest in a concentration-dependent manner, as did KMKKT (Table 2). The sub-G1 apoptotic fraction estimation showed no increase in cell death at the concentrations tested.
Full table
Decursin inhibits AR nuclear translocation and transactivation[14,18]
After androgen binding, AR undergo conformational change and dimerize and translocate from the cytosol (C) to the nucleus (N) to activate gene transcription. The blocking action can be detected in just 1-h pre-treatment in a concentration-dependent manner (Figure 3). These results indicated that decursin rapidly inhibited AR translocation into the N and decreased its protein abundance.
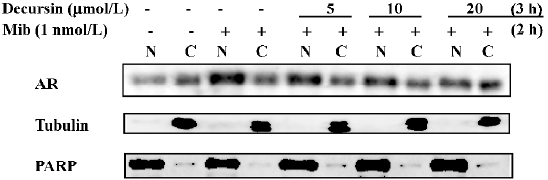
Decursin increases proteasomal degradation of AR[18]
Given that decursin did not decrease the AR mRNA level (Figure 2B), we estimated AR protein degradation after blocking new protein synthesis with cycloheximide. The data showed increased degradation of AR by decursin treatment within 3 h (Figure 4A).
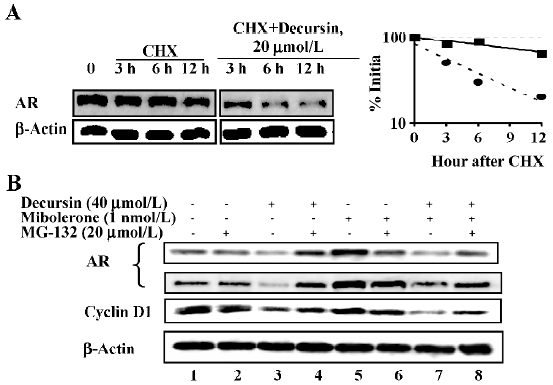
As AR is known to be degraded by 26S proteasome[19], we included the proteasomal inhibitor MG-132 in the absence of androgen and observed a complete block of AR downregulation by decursin (Figure 4B, lane 4 vs lane 3). This inhibitor partially reversed the AR degradation induced by decursin in the presence of androgen (Figure 4B, lane 8 vs lane 7). The abundance changes in cyclin D1, a protein also well known to be degraded through the 26S proteasomal pathway[20], verified the specificity of MG-132. Therefore, these data supported an induction of proteasomal degradation of AR by decursin as one mechanism of downregulating AR protein abundance.
Decursin and AGN extract induce NED, recapitulating the effect of KMKKT[14]
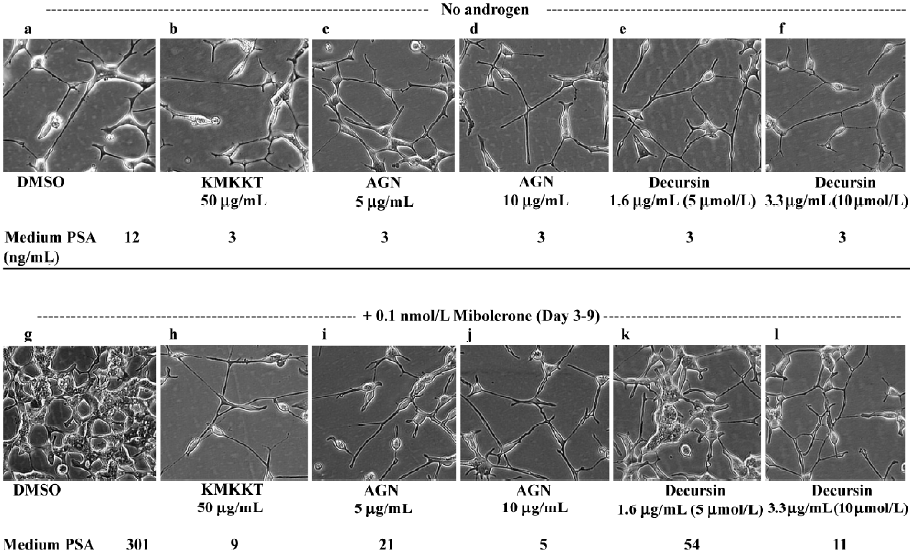
Incubation of sparsely seeded LNCaP cells in phenol red-free medium supplemented with 5% charcoal striped serum (PRFM-CSS) induced NED, which could be reversed by the addition of mibolerone (Figure 5; photographed 9 d after seeding, panel a vs panel g). In the absence of androgen stimulation (panels a–f), decursin (e, f), AGN extract (c, d) and KMKKT extract (b) decreased the basal PSA secretion 4-fold from 12 ng/mL to 3 ng/mL. In the presence of androgen (panels g–l), decursin (k, l) and AGN extract (i, j) inhibited the androgen-stimulated reversal of NED and androgen-stimulated cell proliferation as well as the inhibited PSA secretion in a concentration-dependent manner, achieving a complete block on these parameters with 3.3 µg/mL (10 µmol/L) of decursin and 10 µg/mL of AGN extract, respectively. These data unequivocally support decursin as an anti-androgen and AR compound in AGN and KMKKT extracts.
Comparison of decursin analogs from AGN reveals structure-activity relationships on anti-androgen activity and apoptosis[18]
Other major pyranocoumarin compounds isolated from AGN include decursinol angelate (DA), which is a decursin structural isomer on the side chain, and decursinol, which comprises the pyranocoumarin core but lacks the side chain of the former two[17,21,22] (Figure 1). DA produced nearly identical patterns of suppression on PSA protein (Figure 6A by ELISA and 6B by Western blot analysis). Similar to decursin, DA decreased AR protein expression (Figure 6B) without affecting its mRNA.
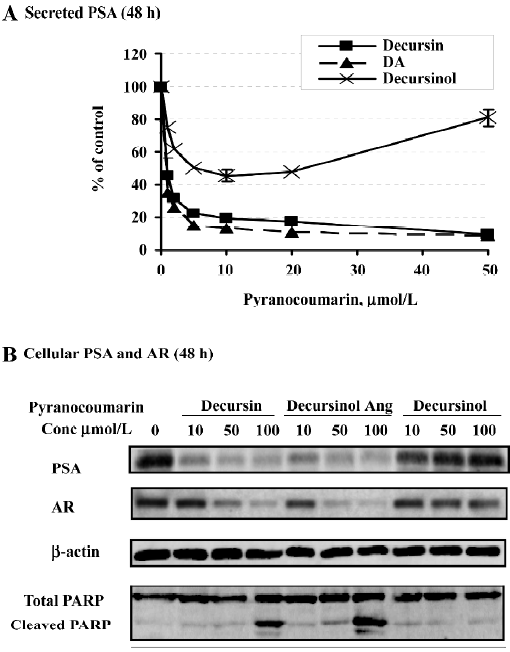
Surprisingly, decursinol exerted biphasic effects with respect to exposure concentration on the cellular and secreted PSA protein levels (Figure 6A, 6B). In particular, at lower micromolar concentrations, decursinol decreased PSA protein in a dose-dependent manner as did decursin and DA (Figure 6A). As the decursinol treatment concentration was increased, PSA expression was not decreased further, instead it exhibited a gradual and concentration-dependent recovery, and was restored to the control cell level with 100 µmol/L decursinol (Figure 6A, 6B). Decursinol did not significantly affect AR protein level (Figure 6B). We have also shown that decursin is a more potent competitor than decursinol for DHT binding to AR[18]. Decursinol did not induce caspase-mediated apoptosis whether decursin and DA did in the same dose range tested (Figure 6B). Therefore, the side chain of decursin and DA is essential for the anti-androgen and apoptotic activities.
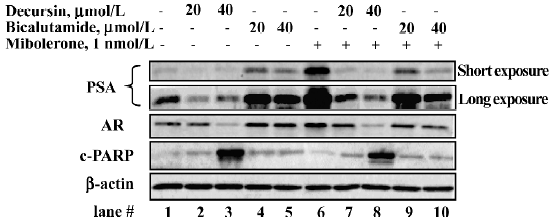
Distinct actions from those of Casodex/bicalu-tamide on PSA and AR protein abundance[14]
We compared decursin in the presence and absence of androgen stimulation with bicalutamide, which is an androgen-binding antagonist[23]. LNCaP cells were cultured in 5% CSS medium for 48 h and exposed to DMSO or indicated concentrations of decursin or bicalutamide for 1 h before the addition of mibolerone for 24 h. As shown in Figure 7, decursin decreased PSA protein as well as AR protein abundance in the absence (lanes 2 and 3 vs 1) and presence of mibolerone (lanes 7 and 8 vs 6). In contrast to decursin, bicalutamide increased the basal levels of cellular PSA as well as AR abundance in the absence of androgen (lanes 4 and 5 vs 1), acting as a partial AR binding “agonist”. As expected by its androgen-binding blocker action, bicaluta-mide decreased PSA protein expression in the presence of mibolerone in a concentration-dependent manner (lanes 9 and 10 vs 6). With respect to LNCaP survival, decursin was more potent than bicalutamide at the same molar concentration of exposure (40 µmol/L) in inducing apoptosis as indicated by the cleaved PARP (lane 3 vs 5; lane 8 vs 10). These results supported distinct novel mechanisms by which decursin inhibited androgen and AR signaling in comparison with bicalutamide.
Persistent inhibition of PSA expression by decursin after removal[14]
Another important feature of decursin is the long-lasting action on androgen and AR signaling. We exposed LNCaP cells for 3 d in complete medium with decursin and then carefully removed the conditioned media and washed the cells twice with serum-free medium. The cells were then fed fresh complete medium for 1, 2, or 3 d. At each time point, the cells were lysed for immunoblot analysis of cellular PSA (Figure 8). Decursin-treated cells maintained a profound suppression of cellular PSA throughout 72 h, whereas the AR protein rebounded to the control level within 24 h after the removal of decursin. These results suggested that decursin exerted a sustained inhibitory action on androgen and AR signaling even after AR protein abundance had fully recovered.

Reported biological and anti-tumor activities of pyranocoumarin compounds
Decursin was first isolated from Angelica decursiva (Fr. et Sav.) in Japan in 1966 and later from Korean AGN[17,24,25]. Geographical origin appears to make a big difference to decursin content as shown by Woo et al[26], who failed to detect decursin in 30 samples of the Chinese Dang-Gui (Angelica sinesis). This was further supported by a more recent study comparing Dang-Gui from Korea, China and Japan[27]. Other major compounds isolated from AGN include DA and decursinol[21,22,28] (Figure 1). Additional minor decursin analogs have recently been reported, including 4'-hydroxytigloyldecursinol, 4'-hydroxydecursin, (2'S,3'S)-epoxyangeloyldecursinol, and (2'R,3'R)-epoxyangeloyl-decursinol[29].
A US patent (United States Patent 6,525,089 Chong et al) was granted in February 2003 to the BiNex company in South Korea as a pharmaceutical with efficacy in ameliorating diabetic hypertension, prevention of renal failure, prevention of diabetic complication, prevention of Cisplatin nephrotoxicity and as a leukemia cell differentiation agent. Decursinol has been studied for its pain suppressing activity in rodent models and for protection against memory loss[30–32]. Other reported activities of decursin and DA include an antibacterial effect[33] and platelet anti-aggregation effect[34]. Some other cellular and molecular actions of decursin include inhibition of lipid droplet accumulation in macrophages[35], blocking of nuclear factor-kappaB activation in macrophages[36], activation of PKC and megakaryocytic differentiation of K562 human erythroleukemia cells[16], and anti-leukemic actions[37].
In terms of the anti-cancer activities, cytotoxic activity of decursin and DA has been described in leukemia cell lines in vitro[15,16,21,37]. A recent report has shown that decursin inhibits the cell cycle and induces apoptosis in LNCaP cells as well as in androgen-independent DU145 and PC-3 PCa cells[38], although the concentrations required for these latter cellular effects were much higher than the concentrations required for the anti-androgen effect[14]. Decursinol was found to be much less active than decursin for cell cycle G1 arrest and apoptosis activity in the DU145 cells. We have discovered partial agonist activity of decursinol in a LNCaP model at high concentrations[18] (Figure 6).
The only published animal study evaluating in vivo anti-cancer activity was reported in 2003[39]. In this study, decursin and DA were found to be active against Sarcoma-180 growth in mice inoculated subcutaneously at a daily intraperitoneal injection dose of 50 or 100 mg/kg body weight. The treatments also prolonged the survival of the sarcoma-bearing mice. It was communicated at the 2007 American Association for Cancer Research (AACR) annual meeting in Los Angeles that orally administered decursin significantly decreased estrogen-independent breast cancer MDA-MB231 xenograft growth in nude mice[40] These data support the oral bioavailability of decursin for in vivo anti-cancer activity. None of these pyranocoumarin compounds has been evaluated for chemoprevention in primary carcinogenesis models in any organ site.
Summary and future work
Our data support decursin and DA as members of a novel class of compounds with potent and long-lasting inhibitory activities against AR signaling in both ligand-dependent and ligand-independent ways[14,18], in addition to their other cellular and molecular actions as summarized above. Decursin and DA do not possess the weak agonist activity of bicalutamide in the LNCaP model system and are more potent than this pure anti-androgen at inhibiting cell growth and survival. The side chain is crucial for the potent and persistent anti-AR activities, and for the cell cycle arrest and apoptosis effects[18]. Aside from the rapid block of AR nuclear translocation, we have identified the following additional mechanisms to account for the specific anti-AR actions: inhibition of binding of DHT to AR and increased proteasomal degradation of AR protein without affecting mRNA level. However, the pharmacokinetics and metabolism of decursin, DA and other pyranocoumarins should be investigated to establish their in vivo stability profiles and metabolites. Studies in appropriate preclinical animal models are needed to establish whether decursin and DA express anti-androgen/AR activity in vivo and whether the anti-cancer activities result from decursin itself or its metabolites.
References
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev 2004;25:276-308.
- Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem 2004;91:483-90.
- Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 2002;62:1008-13.
- Chen S, Song CS, Lavrovsky Y, Bi B, Vellanoweth R, Chatterjee B, et al. Catalytic cleavage of the androgen receptor messenger RNA and functional inhibition of androgen receptor activity by a hammerhead ribozyme. Mol endocrinol 1998;12:1558-66.
- Kuratsukuri K, Sugimura K, Harimoto K, Kawashima H, Kishimoto T. “Decoy” of androgen-responsive element induces apoptosis in LNCaP cells. Prostate 1999;41:121-6.
- Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther 2005;4:505-15.
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. New Engl J Med 2003;349:215-24.
- Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol 2003;21:383-91.
- Young CY, Montgomery BT, Andrews PE, Qui SD, Bilhartz DL, Tindall DJ. Hormonal regulation of prostate-specific antigen messenger RNA in human prostatic adenocarcinoma cell line LNCaP. Cancer Res 1991;51:3748-52.
- Montgomery BT, Young CY, Bilhartz DL, Andrews PE, Prescott JL, Thompson NF, et al. Hormonal regulation of prostate-specific antigen (PSA) glycoprotein in the human prostatic adenocarcinoma cell line, LNCaP. Prostate 1992;21:63-73.
- Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology 2001;142:4795-805.
- Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci USA 1999;96:7490-5.
- Lee HJ, Lee EO, Rhee YH, Ahn KS, Li GX, Jiang C, et al. An oriental herbal cocktail, ka-mi-kae-kyuk-tang, exerts anti-cancer activities by targeting angiogenesis, apoptosis and metastasis. Carcinogenesis 2006;27:2455-63.
- Jiang C, Lee HJ, Li GX, Guo J, Malewicz B, Zhao Y, et al. Potent antiandrogen and androgen receptor activities of an Angelica gigas-containing herbal formulation: identification of decursin as a novel and active compound with implications for prevention and treatment of prostate cancer. Cancer Res 2006;66:453-63.
- Ahn KS, Sim WS, Kim IH. Decursin: a cytotoxic agent and pro-tein kinase C activator from the root of Angelica gigas. Planta Med 1996;62:7-9.
- Kim HH, Ahn KS, Han H, Choung SY, Choi SY, Kim IH. Decursin and PDBu: two PKC activators distinctively acting in the megakaryocytic differentiation of K562 human erythroleukemia cells. Leuk Res 2005;29:1407-13.
- Chi HJ, Kim H.S. Studies on the components of Umbelliferae plants in Korea: pharmacological study of decursin, decursinol and nodakenin. Korean J Pharmacol 1970;1:25-32.
- Guo J, Jiang C, Wang Z, Lee HJ, Hu H, Malewicz B, et al. A novel class of pyranocoumarin anti-androgen receptor signaling compounds. Mol Cancer Ther 2007;6:907-17.
- Sheflin L, Keegan B, Zhang W, Spaulding SW. Inhibiting protea-somes in human HepG2 and LNCaP cells increases endogenous androgen receptor levels. Biochem Biophys Res Commun 2000;276:144-50.
- Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev 1997;11:957-72.
- Ahn KS, Sim WS, Lee IK, Seu YB, Kim IH. Decursinol angelate: a cytotoxic and protein kinase C activating agent from the root of Angelica gigas. Planta Med 1997;63:360-1.
- Sarker SD, Nahar L. Natural medicine: the genus Angelica. Curr Med Chem 2004;11:1479-500.
- Schellhammer PF, Davis JW. An evaluation of bicalutamide in the treatment of prostate cancer. Clin Prostate Cancer 2004;2:213-9.
- Hata K, Sano K. Studies on coumarins from the root of Angelica decursiva FR et SAV. I. The structure of decursin and decursidin. Yakugaku Zasshi 1969;89:549-57.
- Konoshima M, Chi HJ, Hata K. Coumarins from the root of Angelica gigas Nakai. Chem Pharm Bull 1968;16:1139-40.
- Woo YA, Kim HJ, Ze KR, Chung H. Near-infrared (NIR) spectroscopy for the non-destructive and fast determination of geographical origin of Angelicae gigantis Radix. J Pharm Biomed Anal 2005;36:955-9.
- Kim MR, El-Aty AM, Choi JH, Lee KB, Shim JH. Identification of volatile components in Angelica species using supercritical-CO2 fluid extraction and solid phase microextraction coupled to gas chromatography-mass spectrometry. Biomed Chromatogr 2006;20:1267-73.
- Kang SY, Lee KY, Sung SH, Park MJ, Kim YC. Coumarins isolated from Angelica gigas inhibit acetylcholinesterase: structure-activity relationships. Journal of natural products 2001;64:683-5.
- Kang SY, Lee KY, Sung SH, Kim YC. Four new neuroprotective dihydropyranocoumarins from Angelica gigas. J Nat Prod 2005;68:56-9.
- Choi SS, Han KJ, Lee HK, Han EJ, Suh HW. Antinociceptive profiles of crude extract from roots of Angelica gigas NAKAI in various pain models. Biol Pharm Bull 2003;26:1283-8.
- Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, et al. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci 2003;73:471-85.
- Kang SY, Lee KY, Park MJ, Kim YC, Markelonis GJ, Oh TH, et al. Decursin from Angelica gigas mitigates amnesia induced by scopolamine in mice. Neurobiol Learn Mem 2003;79:11-8.
- Lee S, Shin DS, Kim JS, Oh KB, Kang SS. Antibacterial coumarins from Angelica gigas roots. Arch Pharm Res 2003;26:449-52.
- Lee YY, Lee S, Jin JL, Yun-Choi HS. Platelet anti-aggregatory effects of coumarins from the roots of Angelica genuflexa and A. gigas. Arch Pharm Res 2003;26:723-6.
- Ohshiro T, Namatame I, Lee EW, Kawagishi H, Tomoda H. Molecular target of decursins in the inhibition of lipid droplet accumulation in macrophages. Biol Pharm Bull 2006;29:981-4.
- Kim JH, Jeong JH, Jeon ST, Kim H, Ock J, Suk K, et al. Decursin inhibits induction of inflammatory mediators by blocking nuclear factor-kappaB activation in macrophages. Mol Pharmacol 2006;69:1783-90.
- Kim HH, Sik Bang S, Seok Choi J, Han H, Kim IH. Involvement of PKC and ROS in the cytotoxic mechanism of anti-leukemic decursin and its derivatives and their structure-activity relationship in human K562 erythroleukemia and U937 myeloleukemia cells. Cancer Lett 2005;223:191-201.
- Yim D, Singh RP, Agarwal C, Lee S, Chi H, Agarwal R. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res 2005;65:1035-44.
- Lee S, Lee YS, Jung SH, Shin KH, Kim BK, Kang SS. Anti-tumor activities of decursinol angelate and decursin from Angelica gigas. Arch Pharm Res 2003;26:727-30.
- Shin D, Kim H, Jang K, Kim H, Chung H, Kong G. The and anti-tumor effect of decursin in estrogen receptor-negative human breast cancer cells occurs via downregulation of Akt signaling pathway. [abstract]. In: American Association for Cancer Research Annual Meeting: Proceedings; 2007 Apr 14–18; Los Angeles, CA. Philadelphia (PA): AACR; 2007. Abstract nr 447.