Molecular targets of cancer chemoprevention by garlic-derived organosulfides1
Introduction
Health benefits of Allium vegetables including garlic have been noted throughout recorded history, dating back to 1400 BC[1]. The known medicinal benefits of garlic and other Allium vegetables and their constituents include lowering of serum cholesterol level, inhibition of platelet aggregation and increased fibrinolysis [2,3], stimulation of immune function through activation of macrophages and induction of T-cell proliferation[4,5], reduction of blood glucose level[6,7], radioprotection[8], improvement of memory and learning deficit[9,10], protection against microbial, viral and fungal infections[11–13], and anticancer effects[14,15]. Initial evidence for the anticancer effect of Allium vegetables was provided by population-based observational studies[16-18]. For example, You et al[16] documented a significant reduction in gastric cancer risk with increasing intake of Allium vegetables in a population-based, case-control study. Likewise, Steinmetz et al[17] observed an inverse correlation between fruit and vegetable intake and colon cancer risk in the Iowa Women’s Health Study.
The sulfur chemistry of garlic is fairly well understood[19]. The main sulfur compound in intact garlic is γ-glutamyl-S-alk(en)yl-L-cysteine, which is hydrolyzed and oxidized to yield alliin[19]. Alliin accumulates naturally during storage of the bulbs at cool temperature and is the odorless precursor of the organosulfur compounds (OSC) believed to be responsible for the anticancer effect of garlic[20–24]. Processing of garlic bulbs (crushing, cutting or chewing) releases a vacuolar enzyme alliinase that acts on alliin to give rise to extremely unstable and odoriferous compounds, including allicin. Allicin and other thiosulfinates decompose to oil-soluble OSC, including diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), dithiins and ajoene (4,5,9-trithiadodeca-1,6,11-triene-9-oxide)[19].
Preclinical animal studies have indicated that OSC analogues are highly effective in affording protection against cancer induced by a variety of chemical carcinogens[20–24]. For instance, Belman[20] demonstrated that topical application of garlic and onion oil inhibited the incidence of tumor promoted by phorbol–myristate–acetate. Cancer chemoprevention by garlic constituents has been observed against benzo[a]pyrene (BP)-induced forestomach and pulmonary cancer in mice[21], N-nitrosomethylbenzylamine-induced esophageal cancer in rats[22], azoxymethane-induced colon carcinogenesis in rats[23], and 2-amino-1-methyl-6-phenyli-midazo[4,5-b]pyridine-induced mammary tumorigenesis in rats[24]. Elucidation of the mechanisms by which OSC may offer protection against cancer has been a passionate subject of research for the past 20 years. This article summarizes current knowledge on the molecular targets of cancer chemoprevention by garlic constituents.
Modulation of carcinogen activation
Carcinogenic activity of many environmental pollutants (which are usually lipophilic substances) is often dependent on their activation by cytochrome P450-dependent monooxygenases. Garlic constituent DAS and its metabolites diallyl sulfoxide and diallyl sulfone competitively inhibited the activity of cytochrome P-450 2E1 in a time-dependent and NADPH-dependent manner using pseudo-first-order kinetics[25]. Induction of cytochrome P-450 2B1 by treatment with DAS in rat liver microsomes has also been reported[25]. In rats treated with DAS after a 48-h fasting, the starvation-induced hepatic microsomal P-450 2E1 level decreased[26]. Moreover, DAS administration protected against hepatotoxicity caused by exposure to P-450 2E1 substrates, including N-nitrosodimethylamine (NDMA)[26]. The rat nasal cavity is one of the known target organs for carcinogenesis by NDMA, N-nitrosodiethylamine (NDEA) and tobacco carcinogen 4-(methylnitrosamino)1-(3-pyridyl)-1-butanone (NNK)[27]. A single po administration of DAS to male rats caused a significant decrease in the oxidative metabolism of NDEA and NNK in nasal mucosa[27]. Several naturally occurring OSC, including DAS and DADS, inhibited the formation of carcinogenic heterocyclic amines in boiled pork juice (2-amino-3-methyl-imidazo[4,5-f]quinoline, 2-amino-3,4-dimethylimidazo[4,5-f]quinoxaline and 2-amino-3,4-dimethyli-midazo[4,5-f]quinoline)[28]. In contrast, DAS, DADS and DATS have been shown to be inducers of rat liver cytochrome P-450 1A1, 2B1 and 3A1[29]. Collectively, modulation of carcinogen activation may be one of the mechanisms by which garlic constituents may offer protection against chemically induced cancers.
Induction of Phase 2 enzymes and modulation of anti-oxidative enzymes
Experimental evidence exists to suggest that garlic constituents may function as a double-edge sword in the prevention of chemically induced cancers by inhibiting carcinogen activation and enhancing detoxification of activated carcinogenic intermediates through the induction of Phase 2 enzymes, including glutathione transferases (GST) and quinone reductase[30–33]. Wattenberg and colleagues showed that prevention of BP-induced forestomach and lung cancer in mice by garlic OSC was correlated with elevation of hepatic and target organ total GST activity[30]. Studies from our laboratory have shown that DAS, DADS and DATS administration to A/J mice results in induced expression of Alpha (mGSTA3-3, mGSTA1-2, mGSTA4-4), Mu (mGSTM1-1) and Pi class GST (mGSTP1-1) in the liver, lung and forestomach[31–33]. However, OSC-mediated prevention of BP-induced forestomach tumorigenesis, but not lung neoplasia, in A/J mice is most closely correlated with the induction of mGSTP1-1[31,32]. The DAS and DADS were found to be potent inducers of quinone reductase activity and protein level in the forestomach and/or lung of A/J mice[34]. It is interesting to note that DATS administration only moderately increased the activity of quinone reductase in the forestomach or lung (about 1.5-fold increase compared with control mice), despite a marked increase in its protein level, at least in the forestomach[34]. Subsequently, Kong and colleagues showed a positive correlation between OSC-mediated induction of Phase 2 enzymes, activation of anti-oxidant response element and accumulation of transcription factor nuclear factor E2-related factor 2 in HepG2 hepatoma cells[35]. Studies using Clone 9 liver cells documented an essential role for GSTP enhancer I element (GPE I), but not GPE II, in DADS-mediated and DATS-mediated induction of Pi class GST[36]. Garlic-derived OSC have been shown to possess non-enzymatic anti-oxidant activity[37]. The level of reduced glutathione was increased in the liver, lung and/or forestomach by DAS or DATS administration, but not by the non-allylic OSC analogue dipropyl sulfide[38]. These OSC exhibited a differential effect on the activities of glutathione redox cycle enzymes in the liver, lung and forestomach of A/J mice[38]. For instance, a noticeable increase in the activity of glutathione peroxidase relative to control mice was observed only in the lung of DATS-exposed mice[38]. In contrast, Chen et al[39] failed to observe a change in glutathione peroxidase or superoxide dismutase activity in the liver, kidney, lung or brain of rats treated with 50 or 200 mg/kg DAS for 8 or 29 d, although hepatic catalase activity was significantly reduced. DAS and DADS were shown to inhibit N-acetyltransferase activity in a dose-dependent manner in a human colon cancer cell line[40]. Thus, it is reasonable to conclude that the induction of Phase 2 enzymes, especially GST, represents another potential mechanism to explain OSC-mediated prevention of chemically induced cancers. However, the relationship between the chemopreventive effects of OSC and their effects on anti-oxidant enzymes is somewhat inconclu-sive.
Inhibition of post-translational modification of oncogenic Ras
Studies from our laboratory have revealed that oral administration of DADS (8.25, 16.5 and 33 µmol, 3 times per week beginning the day of tumor cell injection), but not its saturated analogue dipropyl disulfide, suppressed growth of H-ras oncogene transformed tumor xenografts in nude mice without causing weight loss or any other side effects[41,42]. The appearance of measurable tumors was also delayed in DADS-treated mice relative to controls[41,42]. The DADS-mediated suppression of H-ras oncogene transformed tumor growth correlated with a decrease in hepatic and tumoral HMG-Co A reductase activity, leading to inhibition of membrane association of p21[41,42]. In contrast, DADS administration did not have any appreciable effect on farnesyltransferase activity in the tumor[41,42]. These studies were the first published reports to document activity of DADS against H-ras oncogene transformed tumors.
Inhibition of cell cycle progression
Cell cycle consists of a series of events involving growth stimulus, replication and division of a eukaryotic cell[43,44]. Cellular stresses may activate signal transduction pathways, referred to as checkpoints, which lead to cell cycle arrest[43,44]. The cell cycle checkpoints ensure completion of phase-specific events and protect against genomic instability or, in cases where the damage is too severe, switch the cell fate to programmed cell death[43,44]. Many anticancer treatments initially cause perturbations in cell cycle progression and the interrupted phase depends on the genetic background of the cell as well as the mode of action of a given treatment. Studies have shown that garlic-derived OSC can suppress growth of cancer cells of different anatomical locations in association with cell cycle arrest, mainly in the G2/M phase of the cell cycle. Milner and colleagues were the first to show that DADS treatment caused dose-dependent and time-dependent accumulation of human colon cancer cells in the G2/M phase of the cell cycle[45,46]. The DADS-mediated G2/M phase cell cycle arrest in human colon cancer cells was accompanied by a decrease in the kinase activity of the Cdk1/cyclin B1 complex, reduction in complex formation between Cdk1 and cyclin B1, and a decrease in Cdc25C protein level[46]. Some of these changes are not specific to colon cancer cells or DADS because similar effects have been reported in other cellular systems with other OSC[47–53]. For instance, DADS (20 µmol/L, 12 h) caused inactivating phosphorylation of Cdk1 in HL-60 cells[47] or decreased Cdk1 level in PC-3 human prostate cancer cells in a dose-dependent manner[48].
We have tried to more thoroughly investigate the mechanism of DATS-induced G2/M phase cell cycle arrest using PC-3 and DU145 human prostate cancer cells as a model[50–53]. DATS was much more effective than either DADS or DAS in causing G2/M phase cell cycle arrest[50]. These results further support the notion that even a subtle change in OSC structure (the oligosulfide chain length) could have a significant impact on its biological activity. Interestingly, a normal prostate epithelial cell line PrEC was resistant to growth inhibition and cell cycle arrest by DATS[50]. The DATS-induced G2/M phase cell cycle arrest in PC-3 cells was associated with increased Tyr15 phosphorylation of Cdk1, inhibition of Cdk1/cyclin B1 activity, increased inhibitory phosphorylation of Cdc25C at Ser216, and downregulation of total Cdc25C protein level[50]. The DATS-mediated hyperphosphorylation and decline in protein level of Cdc25C were abrogated in the presence of anti-oxidants, suggesting a redox-sensitive mechanism for these effects[50]. We showed further that the Ser216 phosphorylation of Cdc25C was mediated by Chk1, although its knockdown by Chk1-specific siRNA was unable to rescue the G2/M phase block caused by DATS[51]. In addition, the DATS-treated PC-3 cells exhibited features characteristic of mitotic arrest, including changes in the tubulin network, chromatin condensation and increased Ser10 phosphorylation of histone H3[51]. Further examination of the DATS-treated PC-3 cells revealed arrest in the prometaphase state that was partially dependent on Chk1 activation and accompanied by accumulation of anaphase promoting complex/cyclosome (APC/C) substrates (cyclin A and cyclin B1), as well as hyperphosphorylation of securin and APC/C components (Cdc20 and Cdh1)[52]. These results indicated that Chk1, which is an intermediary of DNA damage checkpoints[54], may regulate APC/C activity. Mitotic arrest has also been documented for DADS and S-allyl mercaptocysteine (SAMC)[55]. A schematic summary to explain the mechanism of DATS-induced G2/M phase cell cycle arrest in human prostate cancer cells is shown in Figure 1.
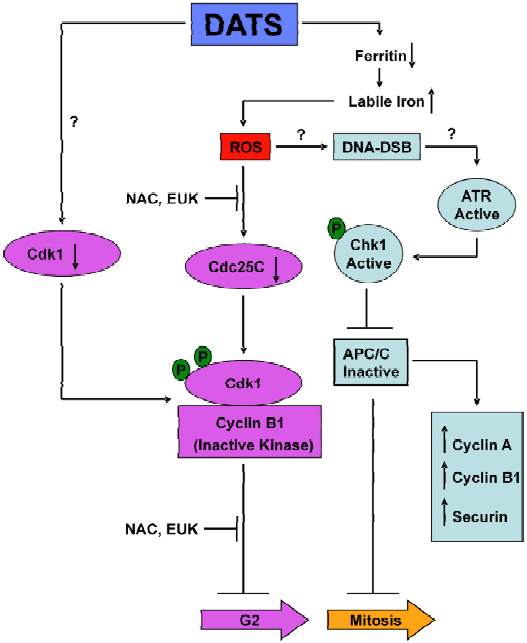
Recent studies from our laboratory have revealed that DATS-mediated cell cycle arrest, at least in human prostate cancer cells, is linked to c-Jun N-terminal kinase (JNK)-dependent generation of reactive oxygen species (ROS)[53]. The DATS-mediated ROS generation appears to be caused by degradation of the iron-storage protein ferritin, which leads to liberation of labile (chelatable) iron[53]. The DATS-mediated degradation of ferritin, an increase in the labile iron pool, ROS generation and the G2/M phase cell cycle arrest are significantly attenuated by genetic suppression of JNK[53].
Several studies show that OSC affect the microtubule network in cancer cells that might initiate mitotic block or apoptosis. For example, treatment of SW480 human colon cancer cells or NIH3T3 mouse fibroblasts with 150 µmol/L water-soluble SAMC caused rapid microtubule depolymerization and cytoskeleton disruption in interphase cells[56]. DATS, but not DADS or DAS, has been shown to induce mitotic arrest in HCT-15 and DLD-1 human colon cancer cells in association with disruption of the microtubule network in interphase cells and inhibition of spindle formation in mitotic cells[57]. This study further revealed DATS-mediated oxidative modification of tubulin β at residues Cys12 and Cys354[57]. Another oil-soluble garlic compound, Z-ajoene, caused G2/M phase cell cycle arrest and disruption of the microtubule network in normal marsupial kidney cells and inhibited tubulin polymerization in vitro[58].
A few reports have also shown that garlic-derived OSC arrest cancer cells in phases other than G2/M. The DADS-mediated suppression of human nosopharyngeal carcinoma cell growth correlated with S phase arrest[59]. Allitridi, synthetic DATS, was shown to arrest human gastric cancer BGC823 cells in the G1 phase and was accompanied by a decrease in cyclin D1 level and an increase in p27 protein level[60]. Nevertheless, inhibition of cell cycle progression appears to be a common cellular response to many structurally diverse OSC.
Histone modification
OSC may affect cancer cell proliferation through modification of histone acetylation and, thus, regulation of gene expression. It has been reported that treatment of DS19 mouse erythroleukemia and K562 human leukemia cells with DADS increases acetylation of histones H4 and H3[61]. DADS and its metabolite, allyl mercaptan, inhibited histone deacetylases in rat hepatoma and human breast cancer cells and it has been suggested that histone acetylation may medi-ate the differentiation process of erythroleukemia cells[61]. Growth inhibitory effects of allicin, SAMC and S-allyl cysteine (SAC) on DS19 cells and SAMC on Caco-2 human colon and T47D human breast cancer cells are correlated with increased histone acetylation[62]. The DADS-induced accumulation of Caco-2 and HT-29 colon tumor cells in the G2/M phase of the cell cycle is correlated with inhibition of histone deacetylase, hyperacetylation of H3 and H4 histones, and upregulation of p21 mRNA and protein level[63,64]. Increase in p21 protein level with treatment of PC-3 cells with DATS has also been documented, but antisense silencing of p21 expression did not have any appreciable effect on DATS-induced G2/M cell cycle arrest[50]. Whether or not p21 induction contributes to DADS-mediated G2/M phase cell cycle arrest remains to be determined.
Induction of programmed cell death (apoptosis)
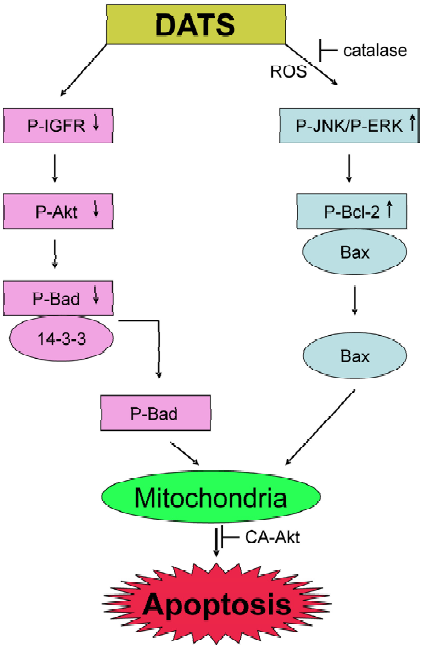
Apoptosis (also known as programmed cell death) is a tightly controlled and evolutionarily conserved process of cellular suicide critical to normal embryonic development and maintenance of tissue homeostasis. Dysregulation of programmed cell death underlies numerous pathological conditions including cancer and, therefore, apoptosis is a valid target in cancer therapy and prevention[65,66]. Garlic-derived OSC have been shown to modulate a number of key elements in cellular signal transduction pathways linked to the apoptotic process. The majority of garlic-derived compounds activate the so called intrinsic or mitochondria-mediated pathway in the execution of apoptosis, which involves loss of mitochondrial membrane potential and release of apoptogenic molecules from the mitochondria to the cytosol[67,68]. Activation of the intrinsic apoptotic pathway is regulated by the Bcl-2 family of anti-apoptotic (eg Bcl-2 and Bcl-xL) and pro-apoptotic (eg Bax and Bak) proteins[69]. Garlic-derived OSC are believed to trigger apoptosis by modulating the levels of Bcl-2 proteins. For example, DAS or DADS treatment increased the ratio of Bax/Bcl-2 in SH-SY5Y neuroblastoma cells, as well as in H460 and H1299 lung cancer cells compared with untreated controls[70,71]. A time-dependent upregulation of Bax protein level and concomitant down-regulation of Bcl-xL protein level was observed in DADS-treated MDA-MB-231 breast cancer cell line[72]. The Z-ajoene-induced apopto-sis in HL-60 cells was associated with caspase-mediated cleavage of Bcl-2[73]. Although Bcl-2 usually acts upstream of the caspase cascade its removal by caspases may amplify the apoptotic signal. Cleavage of Bcl-2 in Z-ajoene-treated cells was inhibited by anti-oxidants, suggesting involvement of ROS in the activation of apoptosis by this agent[73]. Indeed, a dose-dependent and time-dependent increase in the production of peroxide was observed in Z-ajoene-treated HL-60 cells[74]. We have shown that DATS is a more potent inducer of apoptosis in PC-3 and DU145 prostate cancer cells than DAS or DADS[75]. The DATS-induced apoptosis in prostate cancer cells correlates with a decrease in Bcl-2 level as well as with hyperphosphorylation of this protein, which reduces Bcl-2:Bax interaction and activates the mitochondrial pathway of apoptosis[75]. The DATS-mediated hyperphos-phorylation of Bcl-2 in PC-3 and DU145 cells is caused by activation of JNK and, to a lesser extent, extracellular signal-regulated kinase 1/2 (ERK1/2)[75]. Overexpression of Bcl-2 in PC-3 cells conferred statistically significant protection against DATS-induced apoptosis[75]. On the other hand, ectopic expression of Bcl-2 failed to protect against DATS-mediated cell death in LNCaP human prostate cancer cells[76], which unlike PC-3 are androgen responsive and express wild type p53. Whether or not the differential effect of Bcl-2 overexpression on DATS-induced apopto-sis in PC-3 versus LNCaP is related to differences in their androgen responsiveness or p53 status remains to be investigated.
The DATS-induced apoptosis in LNCaP cells correlated with a modest increase in protein levels of pro-apoptotic Bcl-2 family members Bax and Bak[76]. The immortalized mouse embryonic fibroblasts (MEF) derived from Bax and Bak double knockout mice were found to be significantly more resistant to DATS-induced apoptosis compared with the MEF derived from wild-type mice[76]. Consistent with these results, combined knockdown of Bax and Bak conferred statistically significant protection against DATS-induced cell death in LNCaP cells[76]. Furthermore, we showed that DATS-mediated inhibition of PC-3 xenograft growth in nude mice correlated not only with increased apoptosis but also with induction of Bax and Bak proteins in the tumor tissue[77]. However, it is important to point out that Bax and Bak cannot be exclusively responsible for the cell death caused by DATS because combined knockdown of these proteins conferred only partial protection against DATS-induced apoptosis[76]. It is intriguing that DATS treatment causes only a modest increase in protein levels of Bax and Bak, yet knockdown of these proteins confers statistically significant protection against DATS-induced apoptosis[76]. Although the precise mechanism by which Bax and Bak regulate DATS-induced cell death remains elusive, it is possible that DATS treatment causes conformation change and oligomerization of Bax/Bak leading to their translocation to the mitochondria. This possibility is likely based on the following considerations: (1) Bax activation by certain apoptotic stimuli is dependent on ROS generation, which is observed in DATS-treated prostate cancer cells[76]; and (2) microtubule damaging agents have been shown to cause Bax activation, and DATS treatment is known to disrupt the tubulin network[52]. However, further studies are needed to systematically explore this possibility.
We have shown previously that the DATS-induced apoptosis in human prostate cancer cells was, at least in part, regulated by the Akt-Bad pathway[78]. One of the pro-survival functions of Akt (also known as protein kinase B) is to phosphorylate Bad, which causes cytoplasmic sequestration of Bad and consequently protection against interaction with anti-apoptotic Bcl-2 family members. DATS treatment markedly reduced Akt activity in PC-3 and DU145 cells and consequently lowered the phosphorylation of Bad at Ser155 and Ser136, which diminished complex formation between Bad and cytosolic 14-3-3β[78]. Overexpression of constitutively active Akt in PC-3 cells conferred significant protection against DATS-induced apoptosis[78]. The mechanism of DATS-induced apoptosis in human prostate cancer cells is summarized in Figure 2.
Experimental evidence exists to support a critical role of ROS as an intermediary of OSC-induced apoptosis. For instance, DADS-induced apoptosis in HL-60 cells is correlated with ROS generation[79]. The DADS-induced ROS formation in SH-SY5Y neuroblastoma cells is evident as early as 15 min after treatment and is accompanied by oxidation of cellular lipids and proteins[80]. ROS generation in DADS-treated cells was associated with activation of JNK[80]. Over-expression of Cu,Zn-superoxide dismutase or pretreatment with spin trapping molecule 5,5’-dimethyl-1-pyrroline N-oxide offered protection against DADS-induced ROS generation, oxidative damage of cellular macromolecules and apoptosis in SH-SY5Y cells[80].
A few studies have suggested that apoptosis induction by OSC might result from an increase in free intracellular calcium[70,81–84]. Park et al[83] reported a biphasic response for DADS-mediated elevation of calcium level with a rapid peak at 3 min and slow and sustained elevation lasting up to 3 h after the initiation of DADS treatment. The DADS-mediated increase in intracellular calcium level was followed by an increase in hydrogen peroxide level and caspase 3 activation[83]. Recently, it has been shown that both DAS and DADS cause an increase in calcium level in SH-SY5Y cells, which leads to activation of calpain[70]. Calpain is a non-caspase cysteine protease that can contribute to cell death by inducing mitochondria-mediated apoptosis independently of caspases.
Some of the studies cited above have compared apoptotic responses to OSC in cancer cells versus normal cells. Strikingly, malignant cells appear to be more sensitive to OSC-mediated apoptosis than normal non-transformed cells. For example, viability of primary neurons was minimally affected by treatment with 50 or 100 µmol/L DAS or DADS, whereas the neuroblastoma of SH-SY5Y cells treated with these concentrations of DAS or DADS exhibited a marked reduction in cell viability[70]. Similarly, the viability of a normal prostate epithelial line PrEC was not affected by DATS treatment even at concentrations that are highly cytotoxic to prostate cancer cells[50,76]. Finally, Z-ajoene has been shown to cause apoptosis in human leukemia cells, but not in peripheral mononuclear blood cells of healthy donors[74]. The mechanism behind the differential sensitivity of cancer cells and normal cells to apoptosis induction by OSC remains to be elucidated.
Inhibition of angiogenesis and metastasis by garlic constituents
Recent studies using cellular and animal models indicate that garlic extract and its components are able to affect tumor angiogenesis and metastasis. The formation of new blood vessels is necessary for the growth of solid tumors because evidence exists to suggest that tumor growth beyond 1 mm in diameter is restricted by angiogenesis[85]. A study by Matsuura et al[86] showed that aged garlic extract (AGE) suppressed proliferation of transformed human and rat endothelial cell lines and reduced the invasiveness of the endothelial cells by about 20%–30% as assessed by the Matrigel chemoinvasion assay. Additional tests indicated that AGE increased the adhesion of the endothelial cells to collagen and fibronectin in a dose-dependent manner; thus, reducing their motility[86]. Finally, AGE reduced capillary-like tube formation by the endothelial cells in a three-dimensional collagen matrix assay[86]. We have examined the effects of DAS, DADS and DATS on human umbilical vein endothelial cell (HUVEC) viability and have shown that DATS is the most potent of the three analogs in reducing the viability of HUVEC[87]. The DATS-mediated suppression of HUVEC proliferation correlated with caspase 3 and PARP cleavage and apoptotic cell death[87]. The DATS treatment was able to significantly disrupt the capillary-like tube formation and migration by HUVEC that was accompanied by suppression of vascular endothelial growth factor (VEGF) secretion, downregulation of VEGF-Receptor 2 expression, inactivation of Akt and activation of ERK 1/2[87]. In a follow-up study, we found that DATS administration to PC-3 prostate cancer-bearing male nude mice failed to inhibit the formation of new blood vessels in the tumor as judged by immunohistochemical staining for CD31, an endothelial cell marker[77]. Alliin was shown to significantly reduce VEGF and fibroblast growth factor 2- (FGF-2) induced tube formation and angiogenesis in HUVEC and ex vivo in CAM assay[88]. A recent study by Thejass et al[89] showed that DADS and DAS not only inhibited endothelial cell proliferation and migration, but also reduced matrix metalloproteinases 2 and 9. In addition, DAS administered to C57BL/6 mice injected with B16F-10 melanoma cells increased circulating levels of anti-angiogenic factors, tissue inhibitor of metalloproteinase and interleukin-2 levels compared with the untreated animals[90]. Attenuation of cell migration and the induction of cell death by AGE was also documented in rat sarcoma cells[91]. Taylor et al[92] showed that ip injection of ajoene (5–25 µg/g body weight) significantly inhibited pulmonary metastasis in C57BL/6 mice injected with B16/BL6 melanoma cells. Similarly, SAMC administration (300 mg/kg) to CB-17 SCID/SCID mice orthotopically implanted with PC-3 cells reduced the number of lung metastasis per lung by 85.5% and completely abolished adrenal gland metastasis, but had no effect on local metastasis[93]. Based on the reviewed studies it can be concluded that components of garlic extract (in combination or alone) present a great potential as anti-angiogenic and antimetastatic agents.
Concluding remarks
Research over the past 20 years has revealed that garlic-derived OSC can not only inhibit chemically induced cancers but can also suppress growth of cancer cells in culture and in vivo. The garlic compounds appear to target multiple pathways, including the cell cycle machinery, the intrinsic pathway for apoptotic cell death and angiogenic pathway, which may all contribute to their anticancer activities. Future research should focus on clinical assessment of these compounds for prevention/treatment of cancers in humans. A critical question relevant to the clinical development of garlic OSC relates to their plasma or tissue concentration. It remains to be determined whether the micromolar concentrations of OSC needed to inhibit cancer cell growth in culture are achievable in humans. It is important to point out that the peak plasma concentration of DATS in rats following treatment with 10 mg of the compound was shown to be about 31 µmol/L[94]. Although the pharmacokinetic parameters for DATS in humans have not yet been measured, oral administration of 200 mg of synthetic DATS (also known as allitridum) in combination with 100 µg selenium every other day for 1 month to humans did not cause any harmful side effects[95]. It is, therefore, possible that the plasma concentrations of DATS required for cancer cell growth inhibition may be achievable in humans.
Acknowledgements
The authors thank the past (X HU, H XIA, SK SRIVASTAVA, A PAL, J ANTOSIEWICZ, K LEW and Y KIM) and present (D XIAO, H XIAO, E HAHM, S STAN, Y ZENG, SW MARYNOWSKI, J A ARLOTTI and R WARIN) members of the Singh laboratory for helpful discussion and their contributions to the projects on cancer prevention by garlic compounds.
References
- Rivlin RS. Historical perspective on the use of garlic. J Nutr 2001;131:951S-4S.
- Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev 1996;16:111-24.
- Rahman K. Historical perspective on garlic and cardiovascular disease. J Nutr 2001;131:977S-9S.
- Lau BH, Yamasaki T, Gridley DS. Garlic compounds modulate macrophage and T–lymphocyte functions. Mol Biother 1991;3:103-7.
- Lamm DL, Riggs DR. The potential application of Allium sativum (garlic) for the treatment of bladder cancer. Urol Clin North Am 2000;27:157-62.
- Sheela CG, Kumud K, Augusti KT. Anti-diabetic effects of onion and garlic sulfoxide amino acids in rats. Planta Med 1995;61:356-7.
- Augusti KT, Sheela CG. Antiperoxide effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in diabetic rats. Experientia 1996;52:115-20.
- Singh SP, Abraham SK, Kesavan PC. In vivo radioprotection with garlic extract. Mutat Res 1995;345:147-53.
- Moriguchi T, Saito H, Nishiyama N. Aged garlic extract prolongs longevity and improves spatial memory deficit in the senescence-accelerated mouse. Biol Pharm Bull 1996;19:305-7.
- Nishiyama N, Moriguchi T, Saito H. Beneficial effects of aged garlic extract on learning and memory impairment in the senescence-accelerated mouse. Exp Gerontol 1997;32:149-60.
- Cellini L, Di Campli E, Masulli M, Di Bartolomeo S, Allocati N. Inhibition of Helicobacter pylori by garlic extract (Allium sativum). FEMS Immunol Med Microbiol 1996;13:273-7.
- Avato P, Tursil E, Vitali C, Miccolis V, Candido V. Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomed 2000;7:239-43.
- Guo NL, Lu DP, Woods GL, Reed E, Zhou GZ, Zhang LB, et al. Demonstration of the anti-viral activity of garlic extract against human cytomegalovirus in vitro. Chin Med J (Engl) 1993;106:93-6.
- Milner JA. Mechanisms by which garlic and allyl sulfur compounds suppress carcinogen bioactivation. Garlic and carcinogenesis. Adv Exp Med Biol 2001;492:69-81.
- Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets 2003;3:67-81.
- You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, et al. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst 1989;81:162-4.
- Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women’s Health Study. Am J Epidemiol 1994;139:1-15.
- Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G, et al. Allium vegetables and risk of prostate cancer: a population-based study. J Natl Cancer Inst 2002;94:1648-51.
- Block E. The chemistry of garlic and onions. Sci Am 1985;252:114-9.
- Belman S. Onion and garlic oils inhibit tumor promotion. Carcinogenesis 1983;4:1063-5.
- Sparnins VL, Mott AW, Barany G, Wattenberg LW. Effects of allyl methyl trisulfide on glutathione S-transferase activity and BP-induced neoplasia in the mouse. Nutr Cancer 1986;8:211-5.
- Wargovich MJ, Woods C, Eng VW, Stephens LC, Gray K. Chemoprevention of N-nitrosomethylbenzylamine-induced esophageal cancer in rats by the naturally occurring thioether, diallyl sulfide. Cancer Res 1988;48:6872-5.
- Reddy BS, Rao CV, Rivenson A, Kelloff G. Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Res 1993;53:3493-8.
- Suzui N, Sugie S, Rahman KM, Ohnishi M, Yoshimi N, Wakabayashi K, et al. Inhibitory effects of diallyl disulfide or aspirin on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced mammary carcinogenesis in rats. Jpn J Cancer Res 1997;88:705-11.
- Brady JF, Ishizaki H, Fukuto JM, Lin MC, Fadel A, Gapac JM, et al. Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem Res Toxicol 1991;4:642-7.
- Brady JF, Wang MH, Hong JY, Xiao F, Li Y, Yoo JS, et al. Modulation of rat hepatic microsomal monooxygenase enzymes and cytotoxicity by diallyl sulfide. Toxicol Appl Pharmacol 1991;108:342-54.
- Hong JY, Smith T, Lee MJ, Li WS, Ma BL, Ning SM, et al. Metabolism of carcinogenic nitrosamines by rat nasal mucosa and the effect of diallyl sulfide. Cancer Res 1991;51:1509-14.
- Tsai SJ, Jenq SN, Lee H. Naturally occurring diallyl disulfide inhibits the formation of carcinogenic heterocyclic aromatic amines in boiled pork juice. Mutagenesis 1996;11:235-40.
- Wu CC, Sheen LY, Chen HW, Kuo WW, Tsai SJ, Lii CK. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J Agric Food Chem 2002;50:378-83.
- Sparnins VL, Barany G, Wattenberg LW. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis 1988;9:131-4.
- Hu X, Benson PJ, Srivastava SK, Xia H, Gupta V, Zaren HA, et al. Glutathione S-transferases of female A/J mouse liver and forestomach and their differential induction by anti-carcinogenic organosulfides from garlic. Arch Biochem Biophys 1996;336:199-214.
- Hu X, Singh SV. Glutathione S-transferases of female A/J mouse lung and their induction by anticarcinogenic organosulfides from garlic. Arch Biochem Biophys 1997;340:279-86.
- Hu X, Benson PJ, Srivastava SK, Xia H, Bleicher R J, Zaren H A, et al. Induction of glutathione S-transferase pi as a bioassay for the evaluation of potency of inhibitors of benzo(a)pyrene-induced cancer in a murine model. Int J Cancer 1997;73:897-902.
- Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, et al. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun 1998;244:917-20.
- Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, et al. Induc-tion of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med 2004;37:1578-90.
- Tsai C, Yang J, Chen H, Sheen L, Lii C. Garlic organosulfur compounds upregulate the expression of the pi class of glutathione S-transferase in rat primary hepatocytes. J Nutr 2005;135:2560-5.
- Yin M, Hwang S, Chan K. Nonenzymatic antioxidant activity of four organosulfur compounds derived from garlic. J Agric Food Chem 2002;50:6143-7.
- Singh SV, Mack LM, Xia H, Mack LM, Xia H, Gupta V, et al. Differential induction of glutathione redox-cycle enzymes by anti-carcinogenic organosulfides from garlic. Clin Chem Enzym Commun 1997;7:287-97.
- Chen L, Hong JY, So E, Hussin AH, Cheng WF, Yang CS. Decrease of hepatic catalase level by treatment with diallyl sulfide and garlic homogenates in rats and mice. J Biochem Mol Toxicol 1999;13:127-34.
- Chen GW, Chung JG, Hsieh CL, Lin JG. Effects of the garlic components diallyl sulfide and diallyl disulfide on arylamine N-acetyltransferase activity in human colon tumour cells. Food Chem Toxicol 1998;36:761-70.
- Singh SV, Mohan RR, Agarwal R, Benson P J, Hu X, Rudy MA, et al. Novel anti-carcinogenic activity of an organosulfide from garlic: inhibition of H-RAS oncogene transformed tumor growth in vivo by diallyl disulfide is associated with inhibition of p21H-ras processing. Biochem Biophys Res Commun 1996;225:660-65.
- Singh SV. Impact of garlic organosulfides on p21H-ras processing. J Nutr 2001;131:1046S-8S.
- Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif 2000;33:261-74.
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell 2004;116:221-34.
- Knowles LM, Milner JA. Depressed p34cdc2 kinase activity and G2/M phase arrest induced by diallyl disulfide in HCT-15 cells. Nutr Cancer 1998;30:169-74.
- Knowles LM, Milner JA. Diallyl disulfide inhibits p34(cdc2) kinase activity through changes in complex formation and phosphorylation. Carcinogenesis 2000;21:1129-34.
- Tan LM, Zhang MX, Luo HM, Zeng Y, Li J, Cui Z, et al. The initiation of G2/M checkpoint by diallyl disulfide requires the activation of p38 MAP kinase in HL-60 cells. Zhonghua Xue Ye Xue Za Zhi 2004;25:273-6.
- Arunkumar A, Vijayababu MR, Srinivasan N, Aruldhas MM, Arunakaran J. Garlic compound, diallyl disulfide induces cell cycle arrest in prostate cancer cell line PC-3. Mol Cell Biochem 2006;288:107-13.
- Wu CC, Chung JG, Tsai SJ, Yang JH, Sheen LY. Differential effects of allyl sulfides from garlic essential oil on cell cycle regulation in human liver tumor cells. Food Chem Toxicol 2004;42:1937-47.
- Xiao D, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, Zeng Y, et al. Diallyl trisulfide-induced G(2)-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc25C. Oncogene 2005;24:6256-68.
- Herman-Antosiewicz A, Singh SV. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J Biol Chem 2005;280:28519-28.
- Herman-Antosiewicz A, Stan SD, Hahm ER, Xiao D, Singh SV. Activation of a novel ataxia-telangiectasia mutated and Rad3 related/checkpoint kinase 1-dependent prometaphase checkpoint in cancer cells by diallyl trisulfide, a promising cancer chemo-preventive constituent of processed garlic. Mol Cancer Ther 2007;6:1249-61.
- Antosiewicz J, Herman-Antosiewicz A, Marynowski SW, Singh SV. c-Jun NH(2)-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res 2006;66:5379-86.
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 1997;277:1497-501.
- Xiao D, Pinto JT, Gundersen GG, Weinstein IB. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol Cancer Ther 2005;4:1388-98.
- Xiao D, Pinto JT, Soh JW, Deguchi A, Gundersen GG, Palazzo AF, et al. Induction of apoptosis by the garlic-derived compound S-allylmercaptocysteine (SAMC) is associated with microtubule depolymerization and c-Jun NH(2)-terminal kinase 1 activation. Cancer Res 2003;63:6825-37.
- Hosono T, Fukao T, Ogihara J, Ito Y, Shiba H, Seki T, et al. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of beta-tubulin. J Biol Chem 2005;280:41487-93.
- Li M, Ciu JR, Ye Y, Min JM, Zhang LH, Wang K, et al. Antitumor activity of Z-ajoene, a natural compound purified from garlic: antimitotic and microtubule-interaction properties. Carcinogenesis 2002;23:573-9.
- Zhang YW, Wen J, Xiao JB, Talbot SG, Li GC, Xu M. Induction of apoptosis and transient increase of phosphorylated MAPKs by diallyl disulfide treatment in human nasopharyngeal carcinoma CNE2 cells. Arch Pharm Res 2006;29:1125-31.
- Lan H, Lu YY. Effect of allitridi on cyclin D1 and p27(Kip1) protein expression in gastric carcinoma BGC823 cells. Ai Zheng 2003;22:1268-71.
- Lea MA, Randolph VM, Patel M. Increased acetylation of histones induced by diallyl disulfide and structurally related molecules. Int J Oncol 1999;15:347-52.
- Lea MA, Rasheed M, Randolph VM, Khan F, Shareef A, desBordes C. Induction of histone acetylation and inhibition of growth of mouse erythroleukemia cells by S-allylmercaptocysteine. Nutr Cancer 2002;43:90-102.
- Druesne N, Pagniez A, Mayeur C, Thomas M, Cherbuy C, Duee PH, et al. Repetitive treatments of colon HT-29 cells with diallyl disulfide induce a prolonged hyperacetylation of histone H3 K14. Ann N Y Acad Sci 2004;1030:612-21.
- Druesne N, Pagniez A, Mayeur C, Thomas M, Cherbuy C, Duee PH, et al. Diallyl disulfide (DADS) increases histone acetylation and p21(waf1/cip1) expression in human colon tumor cell lines. Carcinogenesis 2004;25:1227-36.
- Kaufmann SH, Gores GJ. Apoptosis in cancer: cause and cure. Bioessays 2000;22:1007-17.
- Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 2005;55:178-94.
- Hengartner MO. The biochemistry of apoptosis. Nature 2000;407:770-6.
- Thornberry N, Lazebnick Y. Caspases: enemies within. Science 1998;281:1312-6.
- Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol 1998;16:395-419.
- Karmakar S, Banik NL, Patel SJ, Ray SK. Garlic compounds induced calpain and intrinsic caspase cascade for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Apoptosis 2007;12:671-84.
- Hong YS, Ham YA, Choi JH, Kim J. Effects of allyl sulfur compounds and garlic extract on the expression of Bcl-2, Bax, and p53 in non small cell lung cancer cell lines. Exp Mol Med 2000;32:127-34.
- Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, et al. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis 2001;22:891-7.
- Li M, Min JM, Cui JR, Zhang LH, Wang K, Valette A, et al. Z-ajoene induces apoptosis of HL-60 cells: involvement of Bcl-2 cleavage. Nutr Cancer 2002;42:241-7.
- Dirsch VM, Gerbes AL, Vollmar AM. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activa-tion of nuclear factor kappaB. Mol Pharmacol 1998;53:402-7.
- Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene 2004;23:5594-606.
- Kim Y, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, et al. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther 2007;6:1599-609.
- Xiao D, Lew KL, Kim Y, Zeng Y, Hahm ER, Dhir R, et al. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res 2006;12:6836-43.
- Xiao D, Singh SV. Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis 2006;27:533-40.
- Kwon KB, Yoo SJ, Ryu DG, Yang JY, Rho HW, Kim JS, et al. Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia HL-60 cells. Biochem Pharmacol 2002;63:41-7.
- Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res 2003;63:5940-9.
- Sundaram SG, Milner JA. Diallyl disulfide inhibits the proliferation of human tumor cells in culture. Biochim Biophys Acta 1996;1315:15-20.
- Sundaram SG, Milner JA. Diallyl disulfide induces apoptosis of human colon tumor cells. Carcinogenesis 1996;17:669-73.
- Park EK, Kwon KB, Park KI, Park BH, Jhee EC. Role of Ca(2+) in diallyl disulfide-induced apoptotic cell death of HCT-15 cells. Exp Mol Med 2002;34:250-7.
- Sakamoto K, Lawson LD, Milner JA. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr Cancer 1997;29:152-6.
- Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med 2003;3:643-51.
- Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, et al. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr 2006;136:842S-6S.
- Xiao D, Li M, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, et al. Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr Cancer 2006;55:94-107.
- Mousa AS, Mousa SA. Angi-angiogenesis efficacy of the garlic ingredient Alliin and antioxidants: role of nitric oxide and p53. Nutr Cancer 2005;53:104-10.
- Thejass P, Kuttan G. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by diallyl disulfide (DADS). Life Sci 2007;80:515-21.
- Thejass P, Kuttan G. Antiangiogenic activity of diallyl sulfide (DAS). Int Immunopharmacol 2007;7:295-305.
- Hu X, Cao BN, Hu G, He J, Yang DQ, Wan YS. Attenuation of cell migration and induction of cell death by aged garlic extract in rat sarcoma cells. Int J Mol Med 2002;9:641-3.
- Taylor P, Noriega R, Farah C, Abad M, Arsenak M, Apitz R. Ajoene inhibits both primary tumor growth and metastasis of B16/BL6 melanoma cells in C57BL/6 mice. Cancer Lett 2006;239:298-304.
- Howard EW, Ling M, Chua CW, Cheung HW, Wang X, Wong YC. Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clin Cancer Res 2007;13:1847-56.
- Sun X, Guo T, He J, Zhao M, Yan M, Cui F, et al. Determination of the concentration of diallyl trisulfide in rat whole blood using gas chromatography with electron-capture detection and identification of its major metabolite with gas chromatography mass spectrometry. Yakugaku Zasshi 2006;126:521-7.
- Li H, Li HD, Wang Y, Xu H, Fan W, Wang M, et al. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin Med J 2004;117:1155-60.
