Insulin-sensitizing effects of a novel α-methyl-α-phenoxylpropionate derivative in vitro1
Introduction
Type 2 diabetes is a chronic disease, often characterized by insulin resistance, which can lead to several secondary complications, such as hypertension, atherosclerosis, coronary artery disease, and hyperlipidemia[1]. Approximately 150 million people worldwide are afflicted with the disease at present, and with a projection of 300 million people being affected by the year 2025, it has become a serious public health problem, particularly in developed countries[2]. Research of an effective antidiabetic agent would be of great interest for the treatment of type 2 diabetes.
Thiazolidinediones (TZD), which are peroxisome proliferator-activated receptor γ (PPARγ) agonists, have been demonstrated to have a variety of clinical effects, including improving insulin sensitivity and glucose tolerance[3,4]. PPAR are ligand-activated transcription factors that belong to the nuclear receptor superfamily. They have specific tissue distribution and play a pivotal role in regulating the expression of a large number of genes involved in glucose and lipid metabolism[5]. PPARγ is mainly distributed in adipose tissue and skeletal muscle, and regulates glucose metabolism. Moreover, PPARγ2 is specific in adipose tissue and essential for adipocyte differentiation[6]. PPARγ agonists could enhance the differentiation of preadipocytes into adipocytes which is relative to their antidiabetic activities[7,8]. Because of the adverse reactions of TZD, such as hepatic toxicity and fluid retention, the research of a non-thiazolidinedione insulin sensitizer has attracted much more attention in recent years[4].
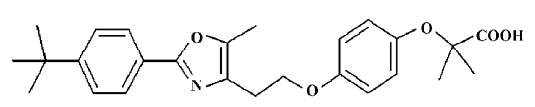
YY20, a novel synthesized non-TZD compound named 2-(3-furan-2-yl-acryloylamino)-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-ethoxy]-phenyl}-propionic acid (Figure 1), was a α-methyl-α-phenoxylpropionate derivative[9,10]. The activation effects of rosiglitazone and YY20 on human PPARγ were tested by using a transient transfection assay. According to the luciferase activity, YY20 activated PPARγ 14±2.4 times to the control at 10 µmol/L, whereas a TZD compound rosiglitazone was 8.7±1.4 times to the control at the same concentration. Although YY20 exhibited potent PPARγ agonist activity, like rosiglitazone in the report gene system, it is essential that the insulin sensitizing effects are proved in the insulin-sensitive cell lines. In this study, the enhancement effects of YY20 on the differentiation of 3T3-L1 preadipocytes into adipocytes were examined and compared with that of rosiglitazone. As adipocyte differentiation markers[6], PPARγ2, glucose transporter-4 (GLUT4), insulin receptor substrate-1 (IRS-1), and adiponectin (ACRP30) expressions were studied in 3T3-Ll preadipocytes treated with YY20. In order to further confirm the insulin-sensitizing effects of YY20, the enhancement effects of insulin-mediated glucose consumption were also investigated in the HepG2 human hepatocellular carcinoma line.
Materials and methods
Materials 3T3-L1 mouse preadipocytes and the HepG2 human hepatocellular carcinoma line were purchased from the Cell Center of the Chinese Academy of Medical Sciences, Shanghai, China. Insulin was the product of Eli-Lilly & Co (Indianapolis, IA, USA); isobutylmethylxanthine (IBMX), dexamethasone (DEX), SR-202, Oil red O, methylthiotetrazole (MTT), and bovine serum albumin (BSA) were purchased from Sigma (St Louis, MO, USA). GLUT4, IRS-1, ACRP30, and the β-actin primary antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Trizol, M-MuLV reverse transcriptase, Taq polymerase, and cell culture mediums were the products of Gibco BRL (Gaithersburg, MD, USA). The PCR primers were synthesized by Sangon (shanghai, China). Enhanced chemiluminescent (ECL) substrate was from Pierce (Rockford, IL, USA). YY20 and rosiglitazone were synthesized in our laboratory and were dissolved in dimethyl sulfoxide (DMSO) to prepare the stock solution.
Adipocyte differentiation assay 3T3-L1 cells were maintained at 37 °C in an atmosphere of 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). The 3T3-L1 preadipocytes were grown in 96-well plates until 2 d postconfluence. The differentiation was induced by addition of 5 µg/mL insulin, 0.5 mmol/L IBMX and 1 µmol/L DEX (INS-IBMX-DEX cocktail). The induction medium was removed 2 d after incubation. After an additional 2 d of incubation in DMEM supplemented with 10% FBS and 5 µg/mL insulin, the medium was changed every other day with DMEM supplemented with 10% FBS. Cells were challenged during the whole period of differentiation with different concentrations of YY20, with rosiglitazone as the positive control and 0.1% DMSO as the vehicle control.
After 7 d differentiation, cells were fixed with 10% formaldehyde for 1 h and then stained with Oil red O (0.1 mg/mL) for 2 h at room temperature. The medium in each well was then removed, and isopropyl alcohol was added to dissolve the precipitate. The optical density (OD) at a wavelength of 510 nm was determined by ELISA spectrometry[11]. Moreover, 3T3-L1 adipocytes treated with YY20 or rosiglitazone for 2 d or for 6 d, together with the vehicle control, were collected for RT-PCR and Western blot analysis.
PPARγ inhibiting assay Two days after confluence, 3T3-L1 cells were treated with 100 µmol/L SR-202 or vehicle for 0.5 h, then 1 µmol/L YY20 or 1 µmol/L rosiglitazone was added. The cells were challenged for 4 d. After that, the cells were cultured in DMEM supplemented with 10% FBS for an additional 2 d and collected for Western blot analysis.
RT-PCR analysis The total RNA from the 3T3-L1 adipocytes treated with increasing concentrations of YY20 or rosiglitazone for 2 d or untreated was isolated using Trizol reagent in accordance with the manufacturer’s instructions. After extraction, mRNA was precipitated by recommended procedures and dissolved in 0.1% diethylpyrocarbonate solution. The RNA content was quantified by using an ultraviolet spectrophotometer at 260 nm. To synthesize first strand cDNA, 7 µL total RNA was incubated with 0.5 µg of oligo (dT) 6 primer and 5 µL deionized water at 65 °C for 15 min. RT of 20 µL was performed with 200 units of M-MuLV reverse transcriptase, 4 µL of 5× reaction buffer (250 mmol/L Tris-HCl; pH 8.3 at 25 °C, 375 mmol/L KCl, 15 mmol/L MgCl2, and 50 mmol/L dithiothreitol) and 1 mmol/L deoxynucleoside triphosphate (dNTP) mixture for 1 h at 42 °C. PCR of 50 µL contained 1 µL of the RT reaction product, 5 µLof 10×PCR buffer (100 mmol/L Tris-HCl, pH 8.3 at 25 °C, 500 mmol/L KCl, and 15 mmol/L MgCl2), 25 units of Taq polymerase, 1 µL of 10 mmol/L dNTP mixture, and 30 pmol of each primer. PCR for the amplification of PPARγ2 was performed with primers (420 bp product; sense: 5'-ATGGGTGAAACTCTGGGA-3', anti-sense: 5'-TCGGCACTCAATGGCCAT-3'), and GAPDH (560 bp product; sense: 5'-ATCTTCTTGTGCAGTGCCAGCC-3', antisense: 5'-GGTCATGAGCCCTTCCACAATG-3') was used as the internal control. Each amplification cycle consisted of 10 s of denaturation at 94 °C, 20 s of annealing at 55 °C, and 30 s of extension at 72 °C. Following amplification, the PCR products were separated by 1.5% agarose gel electrophoresis, stained with ethidium bromide, and visualized under ultraviolet light with a bio-imaging analyzer (Bio-Rad, Hercules, CA, USA).
Western blot analysis 3T3-L1 adipocytes treated with different concentration of YY20 for 6 d or untreated were washed with PBS, collected in RIPA buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 2 mmol/L EDTA, 2 mmol/L EGTA, 25 mmol/L NaF, 25 mmol/L β-glycerolphosphate, pH 7.5, 0.2% Triton X-100, 5 mmol/L EDTA, 1 mmol/L PMSF, 10 µg/mL leupeptin, and 10 µg/mL aprotinin) and lysed for 30 min on ice. Samples were clarified by centrifugation for 30 min at 13 000×g at 4 °C. An aliquot of 40 µg of the supernatant protein from each sample was heated with 4×SDS sample buffer at 95 °C for 5 min, and separated electrophoretically on a 7.5% or 12% SDS-PAGE. Subsequently, proteins were transferred onto a 0.45 µm pore size nitrocellulose membranes for 1 h and blocked for 1 h. Nitrocellulose membranes were then exposed to GLUT4, IRS-1, ACRP30, or the β-actin primary antibody in blocking buffer at 1:500 dilution overnight at 4 °C. Then the membranes were incubated with the anti-rabbit IgG or anti-goat IgG secondary antibody conjugated with horseradish at 1:10 000 dilution for 1 h. The proteins were visualized autoradiographically with ECL, and scanned using a bio-imaging analyzer (Bio-Rad, Hercules, CA,USA).
Glucose consumption assay[12] HepG2 cells were grown in RPMI-1640 (11.1 mmol/L glucose) containing 10% FBS. Two days before the experiments, the cells were plated into 96-well, tissue culture plates with some wells left blank. After the cells reached 80%–90% confluence, the medium was replaced by RPMI-1640 supplemented with 0.2% BSA. Two hours later, the medium was removed and the same culture medium containing YY20, metformin or rosiglitazone, with or without insulin, was added to all wells, including the blank wells. After 24 h of treatment, the medium was removed and its glucose concentrations was determined by the glucose oxidase method[13]. The amount of glucose consumption was calculated by the glucose concentrations of blank wells subtracting the remaining glucose in the cell plated wells.
Following the glucose measuring in the medium, a MTT assay was used to monitor the cell proliferation and to adjust the glucose consumption values[14].
Statistical analysis Data were shown as mean±SD. Differences between individual groups were analyzed by using the t-test. A difference with a P value of <0.05 was considered to be significant.
Results
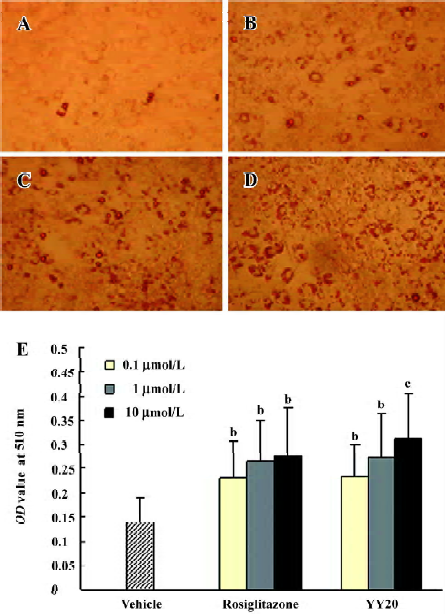
Effect of YY20 on adipocyte differentiation After treatment with YY20 and rosiglitazone at 0.1, 1, and 10 µmol/L in the 3T3-L1 cells during 7 d differentiation, the cells full of lipid droplets increased significantly compared with that of the vehicle control (Figure 2A–2D). By Oil red O staining and OD value determination, it was found that YY20 and rosiglitazone could promote adipocyte differentiation of 3T3-L1 cells significantly (P<0.05, P<0.01; Figure 2E).
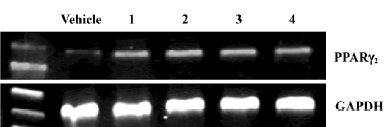
Effect of YY20 on PPARγ2 gene expression To elucidate the pattern of PPARγ2 gene expression after 2 d induction in the 3T3-L1 cells, RT-PCR was analyzed. The results suggested that the expression of PPARγ2 mRNA increased in the cells challenged with YY20, as well as rosiglitazone (Figure 3).
Effect of YY20 on IRS-1 and GLUT4 protein expression The regulation of IRS-1 and GLUT4 expression by increasing concentrations of YY20 was investigated after 6 d of differentiation in the 3T3-L1 cells. Western blot analysis revealed that YY20 could obviously increase the expression levels of IRS-1 and GLUT4 in a concentration-dependent manner (Figure 4A).
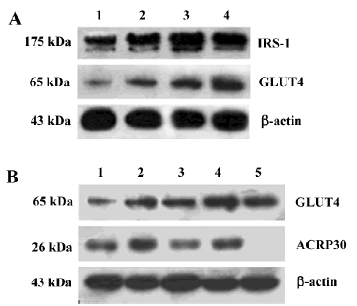
Effect of YY20 on ACRP30 and GLUT4 protein expression, and the influences of the PPARγ inhibitor After 4 d treatment and 6 d differentiation, YY20, as well as rosiglitazone at a concentration of 1 mmol/L, could upregulate the expression of the ACRP30 and GLUT4 protein; this effect could be reversed by the PPARγ specific inhibitor, SR-202. And the increase of ACRP30 expression caused by YY20 was completely reversed by SR-202 (Figure 4B).
Effect of YY20 on glucose consumption After treatment in medium with 11.1 mmol/L glucose for 24 h, a glucose-lowering effect of YY20 was observed. With different levels of insulin added in the culture medium, YY20 exhibited different efficacy of the glucose-lowering effect (Figure 5). When the medium was absent of insulin, the glucose consumption of metformin at 1 mmol/L increased by 59% compared to the vehicle control (P<0.05), whereas there were only 16% and 21% increases by 1 mmol/L of rosiglitazone and YY20, respectively (P>0.05). However, with 1 nmol/L insulin present in the medium, the glucose consumption of cells treated with metformin, rosiglitazone, and YY20 were 75%, 45%, and 63%, respectively (P<0.05); the effect of YY20 was related to the concentration (Figure 6). The effects under 100 nmol/L insulin were similar to that of under 1 nmol/L insulin. According to the MTT results, we found that in the presence of 1 mmol/L metformin and less than 1 mmol/L YY20 or rosiglitazone could not affect cell proliferation after 24 h culture (data not shown). When the glucose consumption values were adjusted with the MTT OD values, the results changed insignificantly.
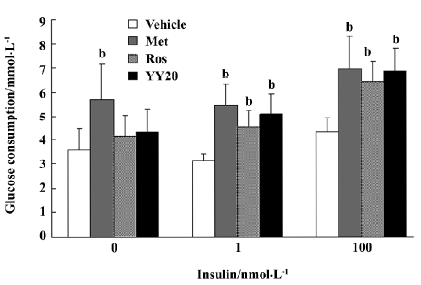
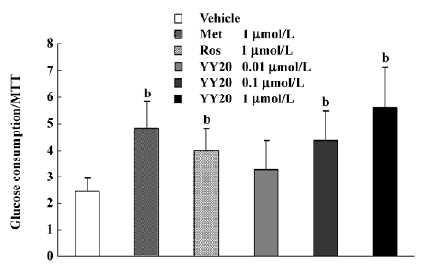
Discussion
It has been widely known that PPARγ agonists are dominant regulators of adipocyte development, and PPARγ activation improves insulin resistance[15–17]. As a TZD, rosiglita-zone is a potent agonist of PPARγ and could significantly improve the adipocyte differentiation in 3T3-L1 cells. Activation of PPARγ can increase the number of small adipocytes, but reduce the number of large adipocytes in white adipose tissues. Because small adipocytes are more sensitive to insulin, an increased number of small adipocytes and a decreased number of large adipocytes in white adipose tissues can alleviate insulin resistance[18]. Furthermore, adipocyte differentiation leads to the expression of adipocyte-specific genes, such as GLUT4 and IRS-1, which are important components of the insulin receptor signal transduction pathway[19–21]. In this study, we found that the novel non-TZD compound YY20 could enhance 3T3-L1 cell differentiation as effectively as rosiglitazone. YY20 could increase lipid accumulation in differentiated cells and upregulate PPARγ mRNA, IRS-1, GLUT4, and ACRP30 protein expression in 3T3-L1 adipocytes, indicating that it might activate PPARγ and enhance insulin sensitivity. Besides an adipocyte diffrentiation mark, ACRP30 is also deemed as a adipocytokine which can improve insulin resistance[22]. In our study, the PPARγ inhibitor could completely reverse the ACRP30 expression upregulated by YY20, but not rosiglitazone, suggesting that the effect of YY20 on ACRP30 expression is more dependent on PPARγ activation than rosiglitazone. What this means to the therapy is still unknown.
Liver, adipose tissue, and skeletal muscles are target tissues of insulin and major sites of glucose and lipid metabolism. In our study, the HepG2 human hepatocellular carcinoma line was used to elucidate the glucose-lowering effect of YY20 in vitro. According to the results, metformin could elevate the glucose consumption of cells regardless of the insulin levels, whereas PPARγ agonist rosiglitazone and YY20 were somewhat dependent on insulin, suggesting the different mechanisms of the glucose-lowering effect between these two kinds of antidiabetic drugs. Since PPARα, not PPARγ, is predominantly expressed in the liver[23], we wonder if the mechanism of the glucose-lowering effect in HepG2 hepocytes is related to the activation of PPARα.
In summary, as a potential activator of PPARγ, YY20 could enhance preadipocyte differentiation and upregulate vital molecules in the insulin signaling pathway, and enhance glucose consumption in HepG2 cells in a concentration- and insulin-dependent manner. These results suggest that further studies should be carried out to develop YY20 as a substitute of TZD for diseases with insulin resistance, such as type 2 diabetes.
References
- Porte D Jr, Schwartz MW. Diabetes complications: why is glucose potentially toxic? Science 1996;272:699-700.
- Seidell JC. Obesity, insulin resistance and diabetes — a worldwide epidemic. Br J Nutr 2000;83 suppl 1:S5-8.
- Boyle PJ, King AB, Olansky L, Marchetti A, Lau H, Magar R, et al. Effects of pioglitazone and rosiglitazone on blood lipid level and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Ther 2002;24:378-96.
- Vasudevan AR, Balasubramanyam A. Thiazolidinediones: a review of their mechanisms of insulin sensitization, therapeutic potential, clinical efficacy, and tolerability. Diabetes Technol Ther 2004;6:850-63.
- Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem 2000;43:527-50.
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994;79:1147-56.
- Kletzien RF, Clarke SD, Ulrich RG. Enhancement of adipocyte differentiation by an insulin sensitizing agent. Mol Pharmacol 1992;41:393-8.
- Choi Y, Kawazoe Y, Murakami K, Misawa H, Uesugi M. Identification of bioactive molecules by adipogenesis profiling of organic compounds. J Biol Chem 2003;278:7320-4.
- Kitajima H, Nakamura M, Goto N. Hybridization of non-sulfonylurea insulin secretagogue and thiazolidinedione-derived insulin sensitizer. Bioorg Med Chem Lett 2000;10:2453-6.
- Brooks DA, Etgen GJ, Rito CJ, Shuker AJ, Dominianni SJ, Warshawsky AM, et al. Design and synthesis of 2-methyl-2-[4-(2-[5-methyl-2-aryloxazol-4-yl]ethoxy)phenoxy]propionic acids: a new class of dual PPARalpha/gamma agonists. J Med Chem 2001;44:2061-4.
- Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantition of adipose conversion and triglycerides by staining intracytoplasmic lipids with oil red O. Histochemistry 1992;97:493-6.
- Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, et al. Effects of berberine on glucose metabolism in vitro. Metabolism 2002;51:1439-43.
- Lott JA, Turner K. Evaluation of Trinder’s glucose oxidase method for measuring glucose in serum and urine. Clin Chem 1975;21:1754-60.
- Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 1989;119:203-10.
- Camp HS, Li O, Wise SC. Differential activation of peroxisome proliferator-activated receptor-gamma by troglitazone and rosiglitazone. Diabetes 2000;49:539-47.
- Vazquez M, Silvestre JS, Prous JR. Experimental approaches to study PPARγ agonists as antidiabetic drugs. Methods Find Exp Clin Pharmacol 2002;24:515-23.
- Lowell BB. PPARγ: an essential regulator of adipogenesis and modulator of fat cell function. Cell 1999;99:230-53.
- Okuno A, Tamemoto H, Tobe K. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese zucker rats. J Clin Invest 1998;101:1354-61.
- Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr 2000;130:S3122-26.
- Wu Z, Xie Y, Morrison RF, Bucher NL, Farmer SR. PPARγ induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPα during the conversion of 3T3 fibroblasts into adipocytes. J Clin Invest 1998;101:22-32.
- White MF, Kahn CR. The insulin signaling system. J Biol Chem 1994;269:1-4.
- Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett 2006;580:2917-21.
- Vidal-Puig AJ, Considine RV, Jimenez-Linan M, Werman A. Peroxisome proliferator-activated receptor gene expression in human tissues. J Clin Invest 1997;99:2416-22.