In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik1
Introduction
Abelmoschus manihot (L) medik is native to the Old World tropics and has been naturalized in some wild New World tropical areas. It is an edible hibiscus of the Malvaceae (Mallow) family, and is also used as a staple in folk medicine in Papua New Guinea, Vanuatu, Fiji, New Caledonia, or China for a variety of purposes, including: the control of fertility, to ease childbirth, to stimulate lactation, to help against menorrhagia, to induce abortion, and to prevent osteoporosis[1–3].
In recent studies, more researchers have been interested in the total flavonoids in the flowers of A manihot. Hyperoside, isoquercetin, and quercetin 3'-glucoside are important ingredients in total flavonoids. Among these, the content of hyperoside is the highest. Hyperoside (hyperin), quercetin-3-O-β-D-galactoside, is a flavonol glycoside which widely exists in many traditional medicines, such as Semen cuscutae[4], St John’s wort[5,6], hawthorn[7], Balbisia calycina[8], and Alchornea cordifolia[9]. As an important bioactive com-pound, hyperoside has been documented to possess antiviral activity[10,11], antinociceptive[12–14], anti-inflammatory[15], cardioprotective[7,16,17], hepatoprotective[18–20], and gastric-mucosal-protective effects[21,22].
In previous studies, it has been shown that hyperoside demonstrates hepatoprotective properties in various chemically-induced hepatocyte injury models[18–20]. However, to our knowledge, the activity of hyperoside against viral hepatitis has never been tested. Therefore, in this study we aimed to evaluate the anti-hepatitis B virus (HBV) activity of hyperoside extracted from A manihot.
Materials and methods
Plant material A manihot were collected from Xinghua, Jiangsu province and identified by Prof Xian-rong WANG at the Anhui Institute of Medical Science (Heifei, China).
Extraction of hyperoside Flower materials were extracted with 80% ethanol and subsequently partitioned in ethyl acetate. Ethyl acetate extracts were chromatographed over a polyamide column using gradient mixtures of ethanol and distilled water. Ethanol extracts yielded total flavonoid following solvent removal under vacuum. The total flavonoid was dissolved in ethanol and crystallized to obtain pure hyperoside (about 97%). The structure of isolate was determined by reverse phase high performance liquid chromatography in comparison with authentic hyperoside (the National Institute for the control of Pharmaceutical and Biological Products, Beijing, China)
In vitro anti-HBV activity tests
Cell culture The HBV-producing 2.2.15 cells were obtained from the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences (Beijing, China). These cultures were derived from HepG2 cells that were transfected with a plasmid vector containing G418-resistance sequences and 2 head-to-tail dimmers of the HBV genome. The cells were found to produce elevated levels of HBeAg and HBsAg. The 2.2.15 cells were cultured in complete MEM (containing 10% FBS, 100 kU/L benzylpenicillin, streptomycin, G-418, L-glutamine 0.03%, pH 7.0) in 75 cm2 tissue culture flasks at 37 ºC in a humidified 5% CO2.
Cell toxicity studies The 2.2.15 cells were first seeded into 96-well plates (Corning Inc, Corning, NY, USA) at a density of 1.0×105 cells per mL and cultured in 200 µL complete MEM containing 10% FBS. After 24 h of incubation, the cells were washed 3 times with phosphate-buffered saline (pH 7.0) and treated with different concentrations (0.20, 0.10, 0.05, 0.025, and 0.0125 g/L) of hyperoside in serum-free medium for 12 d. The medium was replaced every 4 d in MEM supplemented with various concentrations of hyperoside. Untreated cells were used as the control. Because the drug was fuscous, MTT assay could not be used to measure the toxicity of this drug. Therefore, as an index of toxicity, the cell pathological changes (CPE) were observed by a microscope. The degree of CPE was graded as: all positive cells (–), the number of negative cells <25% (+), 25%–49% (++), 50%–75%(+++), and >75% (++++). This test was done 3 times under the same conditions.
Determination of HBsAg and HBeAg The 2.2.15 cells were incubated in 24-well plates at a density of 1.0×105 cells per mL in 1000 µL MEM medium containing 10% FBS. After 24 h, the 2.2.15 cells were treated with different concentrations of hyperoside (0.05, 0.025, 0.0125, 0.00625, and 0.003125 g/L) in serum-free medium. The cells were grown in the presence of hyperoside for 9 d and the medium was replaced every 3 d. After d 6 and d 9, the supernatant was collected and performed at -20 °C. The HBsAg and HBeAg in the culture medium were simultaneously measured by EIA kits on d 6 and d 9. This test was done twice under the same conditions.
In vivo anti-HBV activity tests
Animals and treatments Peking ducklings within 1 d of hatching were used as the in vivo model system. The animals were obtained from an animal breeding farm, Chinese Academy of Medical Sciences, [SCXK-(Peking)2002–001, Peking, China]. The animal quarters were maintained at 22±2 °C and 50%±10% humidity with a 12 h light/12 h dark cycle. The ducklings were inoculated intravenously with duck hepatitis B virus (DHBV)-DNA-positive serum from the Shang-hai ducks (0.2 mL/animal). Seven days after the injection, the ducklings were divided into 5 groups: the control group (normal saline); the positive drug group (lamivudine or 3TC, 0.05 g·kg-1·d-1); and the hyperoside 0.02, 0.05, and 0.10 g·kg-1·d-1 groups. Drugs were administered orally twice daily for 10 d. Sera were obtained before treatment (d 0), on d 5 and d 10 during treatment, and d 3 (d 13) after the cessation of treatment. The serum levels of DHBV-DNA were detected by dot hybridization.
Detection of DHBV-DNA Fifty μL of serum was spotted directly onto the nitrocellulose filters. DNA hybridization was initiated by adding a recently prepared DHBV 32PDNA probe at 1.0×106 cpm/mL using the same prehybridization procedure over night. Filters were washed twice in 1×SSC (20×SSC: 3 mol/L NaCl, 0.3 mol/L sodium citrate, pH 7.0), 0.1% SDS at 65 °C for 2 h, and 1×SSC at room temperature for 30 min with gentle, constant agitation. The filter was dried and autoradiographed at -70 °C using X-ray film with an enhancer screen. After an autoradiographic image had been obtained, the filter was exposed in the phosphorimaging screen for 1–2 h, and the samples were quantitated by FujixBAS1000 (Fuji, Tokyo, Japan); the percentage density of the phosphorimaging units was calculated.
Histopathological examination of hepatocytes On d 13, each duckling was laparotomized to obtain the liver immediately after collecting blood from the leg vein. Fragments of the ducklings liver were fixed in 10% formalin solution, dehydrated with ethanol solution from 50% to 100%, embedded in paraffin and cut into 5 µm sections, and stained using hematoxylin-eosin dye for photomicroscopic observations.
Statistical analysis All data were expressed as mean±SD and analyzed by one-way repeated-measure ANOVA and t-test for comparisons between groups. Values were considered significantly different at P<0.05.
Results
In vitro anti-HBV activities
Cytotoxicity of hyperoside in 2.2.15 cells Hyperoside-induced cytotoxicity was observed by microscope. After 12 d of incubation with 0.05 g/L hyperoside, no significant difference was found from that of the control. However, when the hyperoside concentration increased, cell injury caused by hyperoside was observed. According to the Reed-Meuench equation, the 50% toxic concentration (TC50) was 0.115 g/L, and the maximum nontoxic concentration (TC0) was 0.05 g/L (Table 1).
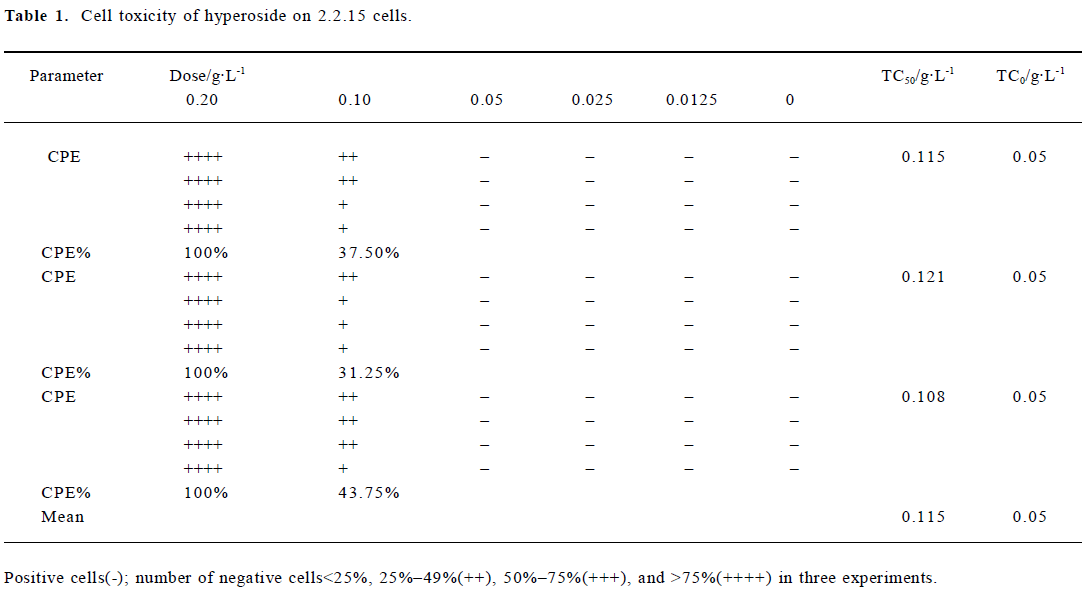
Full table
Inhibitory effect of hyperoside on HBsAg and HBeAg expression in 2.2.15 cells After 8 d of incubation, HBsAg and HBeAg produced in the culture medium were measured. The positive control drug was 3TC (0.05 g/L). The results showed that HBeAg and HBsAg of the cells incubated with hyperoside were less than that of the control cells, and the median effective concentrations (IC50) were about 0.012 and 0.015 g/L, respectively, on d 4, 0.009 and 0.011 g/L, respec-tively, on d 8 (Tables 2, 3). The therapeutic indices, determined by IC50 vs TC50, were about 9.58, 12.78 of HBeAg, respectively, on d 4 and d 8 and 7.67 and 10.45 of HBsAg on d 4 and d 8, respectively (data not shown). The HBeAg inhibition rates of 3TC were 60.54% on d 4 and 54.68% on d 8, respectively (data not shown). The HBsAg inhibition rates of 3TC were 58.23% on d 4 and 41.48% on d 8 (data not shown). Table 2 shows that significant inhibition of HBeAg by hyperoside was observed at 0.0125 g/L (P<0.01), and a high inhibition was noted at the hyperoside concentration equal to 0.05 g/L on d 8. Furthermore, hyperoside also showed inhibitory activity on HBsAg excretion, about 82.27% at the concentration of 0.05 g/L on d 8. The inhibition rate percentages of both HBeAg and HBsAg were both time- and dose-dependent. It could also be observed that hyperoside had a relatively stronger inhibition on HBeAg than HBsAg.
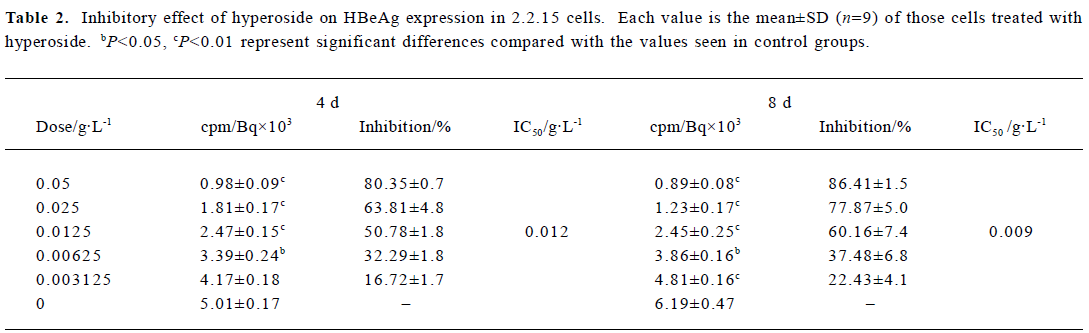
Full table
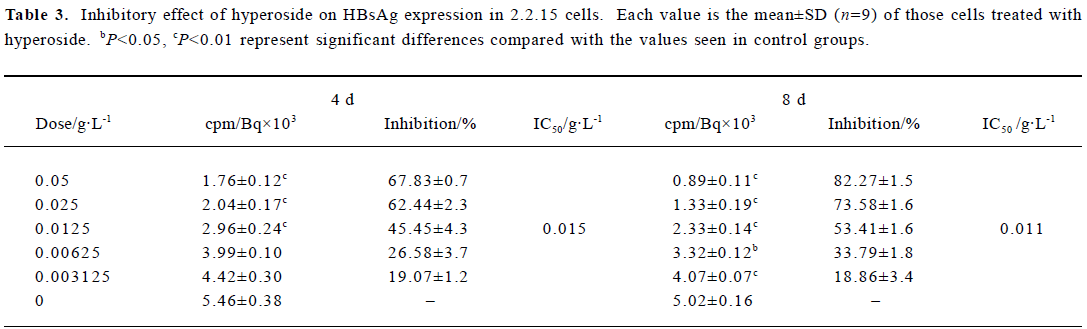
Full table
In vivo anti-DHBV activities
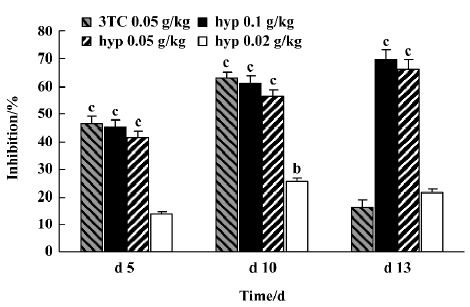
Inhibitory effect of hyperoside on DHBV-DNA Next, the anti-HBV activity of hyperoside was investigated in vivo using the DHBV-DNA-infected duckling model. During this experimental period, no significant side effects were observed in animals receiving antiviral therapy or in control animals. The levels of serum viral DNA were recorded in the 5 groups before the experiment (d 0). As shown in Table 4, with the exception of the control group, serum levels of DHBV-DNA of each group decreased with different extents after treatment with hyperoside and 3TC on d 5 and d 10, respectively. Among these, the hyperoside 0.10 g·kg-1·d-1 group, the 0.05 g·kg-1·d-1 group, and the 3TC group showed a significant decrease of DHBV-DNA (P<0.01). Three days after the cessation of treatment with 3TC, the viral replication level returned to the pretreatment baseline. In the ducks treated with hyperoside, the effect of DHBV-DNA inhibition lasted (Figure 1). The mean percentage inhibition of viral DNA levels with hyperoside 0.10 and 0.05 g·kg-1·d-1 was 60.94% and 56.24%, respectively, on the last treatment day (d 5).
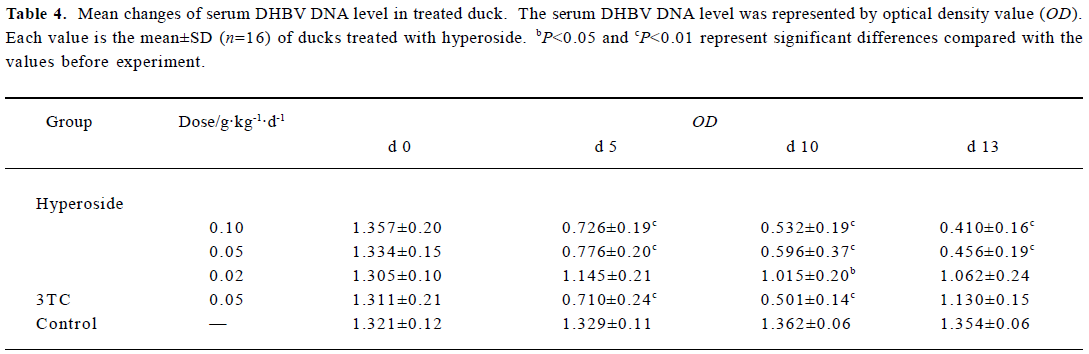
Full table
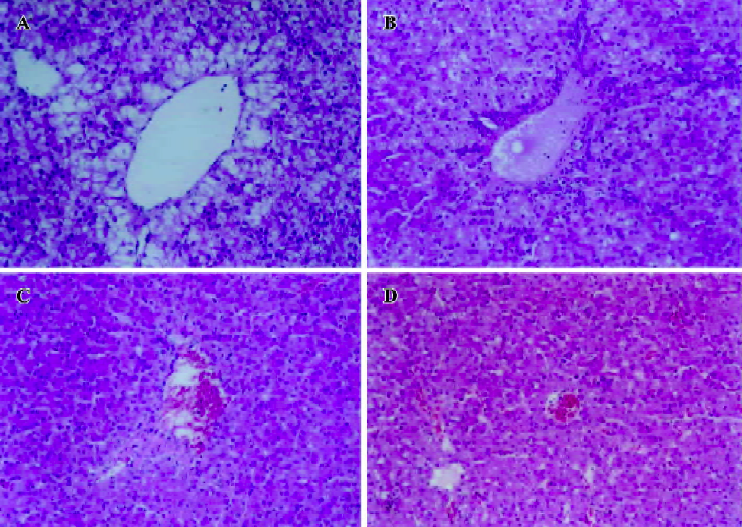
Histopathological features Histopathological profiles of the liver from the model group ducklings revealed necrosis, steatosis, and often swelling of the hepatic cytoplasm. The protective effect of hyperoside was confirmed by histopathological examinations. Administration of hyperoside to the experimental animals (0.10 g·kg–1·d–1) showed a significant improvement of the hepatocellular architecture over the model group, as evident from a considerable reduction in necrosis and vacuolation (Figure 2).
Discussion
Hepatitis B is a major epidemic disease in South-East Asia, China, and Africa, where approximately 10% of the population are chronic carriers[23]. HBV replicates within infected hepatocytes and expresses viral epitopes on them to induce T-cell mediated immune responses to cause hepatitis. Currently, there are 2 arms of therapy to manage chronic active hepatitis B: direct antiviral therapy to inhibit replication of HBV or indirect immunomodulatory therapy to enhance cellular immunity to destroy the virus-infected hepatocytes.
Due to the low efficiency and many limitations of immunomodulatory therapy with IFN-α, direct antiviral therapy could have increasing importance. However, although direct antiviral therapy with lamivudine could efficiently control chronic active hepatitis B, drug resistance could develop progressively after 6–9 months of the initiation of therapy[24,25]. These unsatisfactory therapeutic results strengthen the need for new anti-HBV agents. In this report, our results imply that hyperoside possesses anti-HBV activity.
In our experiment, the data shows that the TC50 of hypero-side was 0.115 g/L and the TC0 was 0.05 g/L in 2.2.15 cells, which suggests that the inhibitory activity of hyperoside had no cytotoxicity. In the nontoxic concentration, hypero-side inhibited HBeAg and HBsAg in a dose- and time-dependent manner. Nevertheless, hyperoside showed stronger inhibition on HBeAg with IC50=0.012 g/L than HBsAg with IC50=0.015 g/L on d 4. At 0.05 g/L, the inhibition rate percentage of hyperoside on HBeAg and HBsAg in 2.2.15 cells were 86.41% and 82.27%, respectively. Recent research showed that another flavonoid from Phyllanthus urinaria ellagic acid effectively blocked HBeAg secretion (IC50=0.07 mg/mL). However, compared to hyperoside, it had no effect on HBsAg[26].
As an HBV-infected animal model, DHBV-infected Peking ducks were also used to evaluate the effects of hyperoside against HBV. At 0.05 and 0.10 g·kg-1·d-1, hypero-side significantly decreased DHBV replication with the inhibition rate percentage 65% and 70%, respectively, on d 10. On d 13, 3TC showed a rebound effect because of drug cessation like most antiviral drugs. However, in contrast with 3TC, the inhibition rate percentage of hyperoside on HBV-DNA showed no rebound after cessation on d 13. It indicated that hyperoside could maintain for a long time in treating viremia of HBV, and the effect of DHBV-DNA inhibition showed a concentration-dependent response. From histopathological examination, we could also confirm the protective effect of hyperoside on the livers of DHBV-infected ducklings. In summary, our results show that hyperoside possesses anti-HBV activity whether the tests were done in vivo or in vitro.
It is necessary to reveal the possible mechanisms of the anti-HBV activity of hyperoside. During the replication of hepadnaviruses DNA, DNA polymerase was a target enzyme of antihepatitis drugs. Therefore, further investigation of the DNA polymerase level in HBV replication is required. Xiong et al[18] used the in vitro D-GalN/TNF-α model to confirm that many flavonoids, including hyperoside, protect liver cells from TNF-α-induced hepatocyte apoptosis. TNF-α has been found to be a very important pathogenic mediator in patients with alcoholic liver disease and viral hepatitis, as well as in many animal liver injury models[27]. Recent research showed that the activation of the annexin A7 (Axn7) gene and the expression of the Axn7-GFP fusion protein could cause a decrease in HBsAg secretion[28]. However, the precise mechanism of the anti-HBV activity of hyperoside needs further investigation.
Acknowledgement
We thank Dr Li ZHUANG (the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences, Beijng, China) for his excellent technical supports in this study.
References
- Bourdy G, Walter A. Maternity and medicinal plants in Vanuatu. I. The cycle of reproduction. J Ethnopharmacol 1992;37:179-96.
- Preston SR. Aibika/Bele- (L.) Medik. In: CAB ABSTRACTS. CAB International. 1998. p97.
- Puel C, Mathey J, Kati-Coulibaly S, Davicco M J, Lebecque P, Chanteranne B, et al. Preventive effect of Abelmoschus manihot(L.) Medik. on bone loss in the ovariectomised rats. J Ethno-pharmacol 2005;99:55-60.
- Ye M, Li Y, Yan Y, Liu H, Ji X. Determination of flavonoids in Semen Cuscutae by RP-HPLC. J Pharm Biomed Anal 2002;28:621-8.
- Pietta P, Gardana C, Pietta A. Comparative evaluation of St. John’s wort from different Italian regions. IL Farmaco 2001;56:491-6.
- Sloley BD, Urichuk LJ, Ling L, Gu LD, Coutts RT, Pang PK, et al. Chemical and pharmacological evaluation of Hpericum perforatum extracts. Acta Pharmacol Sin 2000;21:1145-52.
- Zhang Z, Chang Q, Zhu M, Huang Y, Ho WK, Chen Z. Characterization of antioxidants present in hawthorn fruits. J Nutr Biochem 2001;12:144-52.
- Mino J, Acevedo C, Moscatelli V, Ferraro G, Hnatyszyn O. Antinociceptive effect of the aqueous extract of Balbisia calycina. J Ethnopharmacol 2002;79:179-82.
- Manga HM, Brkic D, Marie DE, Quetin-Leclercq J. In vivo anti-inflammatory activity of Alchornea cordifolia (Schumach.& Thonn.) Mull Arg (Euphorbiaceae). J Ethnopharmacol 2004;92:209-14.
- Shahat AA, Pieters L, Apers S, Nazeif NM, Abdel-Azim NS, Berghe DV, et al. Chemical and Biological Investigations on Zizyphus spina-christi L. Phytother Res 2001;15:593-7.
- Shi Y, Shi RB, Liu B. Studies on antiviral flavonoids in Yinqiaosan Powder. Chin J Chin Mat Med 2001;26:320-2. Chinese..
- Chen ZW, Ma CG, Fang M. Mechanism of analgesic action of hyperin. Acta Pharm Sin 1989;24:226-30. Chinese..
- Ma CG, Xiang ZM, Xu SY. Mechanism of analgesic action of hyperin on centrum. Chin Pharmacol Bull 1991;7:345-9. Chinese..
- Zhang JS, Chen ZW, Wang Y, Song BW, Dong LY, Cen DY. Mechanism of analgesic action of hyperin on spinal cord. Anhui Med 1998;19:3-5. Chinese..
- Lee SJ, Son KH, Chang HW, Do JC, Jung KY, Kang SS. Anti-inflammatory activity of naturally occurring flavone and flavonol glycosides. Arch Pharm Res 1992;16:25-8.
- Li QL, Yu GX, Chen Z, Ma CG. Inhibitory mechanism of hyperin of the apoptosis in myocardial ischemia/reperfusion in rats. Acta Pharm Sin 2002;37:849-52. Chinese.
- Wang WQ, Ma CG, Xu SY. Protective effect of hyperin against myocardial ischemia and reperfusion injury. Acta Pharmacol Sin 1996;17:341-4.
- Xiong Q, Fan W, Tezuka Y, Adnyana IK, Stampoulis P, Hattori M, et al. Hepatoprotective effect of Apocynum venetum and its active constituents. Planta Medica 2000;66:127-33.
- Carlo GD, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci 1999;65:337-53.
- Ito M, Shimura H, Watanaba N, Tamai M, Hanada K, Takahashi A, et al. Hepatoprotective compounds from Canarium album and Euphorbia nematocypha. Chem Pharm Bull (Tokyo) 1990;38:2201-3.
- Zhao WZ, Chen ZW, Song BW., Wang YL, Wang Q, Fang M, et al. The protective effect of hyperin on gastric mucosal injury in mice and its mechanism. Acta Anhui Med Univ 1999;3:178-80.
- Song BW, Chen ZW, Ma CG. The cell protective effect of hyperin on gastric mucosal injury in rats. Chin Pharmacol Bull 1995;11 Suppl:46-8. Chinese..
- Kane M. Global programme for control of hepatitis B infection. Vaccine 1995;13 Suppl 1:S47-9.
- Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterol 2000;119:172-80.
- Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, et al. Extented lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001;33:1527-32.
- Shin MS, Kang EH, Lee YI. A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antivir Res 2005;67:163-8.
- Bradham CA, Plumpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity I. TNF-induced liver injury. Am J Physiol 1998;275:G387-92.
- Lam WY, Leung KT, Law PT, Lee SM, Chan HL, Fung KP, et al. Antiviral effect of phyllanthus nanus ethanolic extract against Hepatitis B virus (HBV) by expression microarray analysis. J Cell Biochem 2006;97:795-812.
