Non-cytolytic antigen clearance in DNA-vaccinated mice with electro-poration1
Introduction
Hepatitis B virus (HBV) infection remains a serious worldwide health problem[1]. Approximately 5%–15% of infected adults and 90%–95% of infected newborns fail to clear infection and become chronic carriers who would face greater risk of developing cirrhosis and hepatocellular carcinoma later in life[2]. Although prophylactic vaccination using the recombinant HBV surface antigen was introduced more than 20 years ago and has been a great success, existing carriers still account for up to 20% of the population in certain Asian and African countries[3]. Therefore, there is an urgent need for new and effective therapeutic approaches to treat these patients. The success of prophylactic vaccine brought high hopes towards the development of immunotherapy approaches against existing viral infection to complement the current interferon treatment and antiviral chemotherapy. Agents such as the hepatitis B (HB) subunit vaccine, cytotoxic T lymphocyte (CTL) epitope vaccine, Hepatitis B vaccine and anti-HBs complex all have been under investigation for such purposes[4–6].
Among all the approaches, DNA-based vaccination agents seem most promising for their ability to elicit both strong humoral and cellular immunities[7,8]. Further formulation and delivery methods are being developed to augment the immunogenicity, such as using viral vectors[9], micros-pheres[10], gene gun[11], or electroporation (EP)[12,13]. We adopted the EP-mediated delivery approach and showed that the EP-mediated HBV surface antigen (HBsAg) encoding DNA vaccination resulted in much higher antibody titers and stronger CTL responses in healthy animals and non-human primates[14].
To evaluate the therapeutic potential of such a vaccination approach, it would be more important to demonstrate viral clearance resulting from the treatment. Several earlier studies used a transgenic mouse model containing the HBV genome as the relevant disease model[15]. However, since the HBV genes were expressed at low levels throughout the animal and not limited in the liver tissues as in a real human infection, transgenic mice are not considered suitable for evaluating antiviral drugs or therapeutic methods, especially when observing the clearance process histologically in the liver tissues. In this study, we developed a model system in which liver cells were transfected and made to express a large quantity of HBsAg using the hydrodynamic DNA injection method to test the clearance ability of immune response induced by EP-mediated HBV DNA vaccination[16,17]. We showed that not only the secreted viral proteins, but also those in the cytoplasm of hepatocytes, were quickly eliminated, without apparent cytotoxicity in EP-mediated vaccination animals.
Materials and methods
Plasmid and animals Plasmid DNA encoding the HBV surface antigen preS2-S[18] was constructed using the pcDNA3.1 vector as described previously and named pHBS[19]. Recombinant hepatitis B vaccine (rec-HBs) was purchased from Kangtai Biological Products (Shenzhen, China). Healthy female BALB/c mice, 6–8 weeks old, were purchased from Fudan University Animal Facility (Shanghai, China), where they were housed in compliance with the regulations of the China Council for Animal Care. Their use in this experiment and all the experimental procedures conformed to the guideline of the Shanghai Experimental Animals Management.
Animal immunization The mice were anaesthetized and shaved to expose the injection site above the gastrocnemius muscle on 1 hind leg; then were administered the injections of 5 µg pHBS or 0.5 µg rec-HBs. For the EP-mediated DNA vaccination (pHBS/EP), the mice were treated with EP within 1 min after the injection using the following parameters: 60 V/cm field strength, 6 pulses, and 50 ms duration with a 1 s interval between pulses.
Hydrodynamic transfection 10 µg HBV surface antigen expression plasmid in sterile saline in a volume equal to 10% of mice body weight was rapidly injected into tail vein.
Measurement of HBsAg level and anti-HBs titers Blood samples were collected at specific time points and sera were isolated for analysis. For the muscle and liver samples, the mice were killed at designated time points and the whole liver or entire muscle fiber was removed and homogenized in cell lysis solution. The amount of HBsAg and the HBV surface antibody (anti-HBs) titers was measured using the corresponding ELISA kits (Sino-American Biotechnology, Shanghai, China). The HBsAg standards in µg/mL and anti-HBs standards in mIU/mL were also purchased from the Sino-American Biotechnology Company. The antigen level and antibody titers were calculated based on the standard curve obtained.
ELISpot assays The cellular immune responses of the mice after vaccination were tested with an ELISpot assay using the IFN-γ ELISpot kit (R&D Systems, Minneapolis, MN, USA). Spleen cells were isolated 2 weeks after vaccination and washed 3 times in RPMI medium, then plated into the blocked ELISpot plate at the density of 1×106 cells per well. The HBsAg mouse epitope peptide with the sequence of LTKILTIPQSLDSWWTSLN was synthesized by Shenergy Biocolor Bioscience and Technology (Shanghai, China) for antigen-specific stimulation. The ELISpot assay was carried out according to the manufacturer’s protocol. The blue spots in each well were counted manually under a dissection microscope.
Immunohistochemical assay of HBsAg expression in the liver At a specific time after the hydrodynamic injection, the mice were killed and the liver portions were immediately fixed in 10% buffered formaldehyde, embedded in paraffin, sectioned at 5 µm thickness, and mounted on slides. For the HBsAg immunohistochemical assay, tissue sections were incubated for 10 min with 3% (v/v) H2O2 and blocked with 5% (w/v) bovine serum albumin in PBS for 10 m at 20–25 oC. Goat anti-HBs (dilution 1:1000; Dako, Carpinteria, CA, USA) was then added and kept incubated for 1.5 h at room temperature. After being washed, the sections were incubated with the biotinylated secondary antibody, followed by a streptavidin-horseradish peroxidase complex in accordance with the manufacturer’s protocol (LSAB staining kit, Dako, USA). Finally the reactions were visualized in a solution containing 3, 3'-diaminobenzidine tetrahydrochloride and Meyer’s hematoxylin counterstaining. The surface antigen-positive cells were counted from 4 different views of the liver sections from 6 different animals in each group.
Histological examination of the liver tissues The tissue sections were prepared according the method mentioned above, and the histological changes were examined by HE staining.
Alanine aminotransferase assay Serum alanine aminotransferase (ALT) levels were measured at various time points using an alanine aminotransferase kit (Fosun Long March Medical Science, Shanghai, China) and an ANALYTECH-738PLUS biochemical analyzer (ANTAI Diagnostics, Shanghai, China).
Statistical analysis Unless otherwise stated, the results are presented as mean±SD. Statistical analyses were made using Student’s t-test.
Results
Improved immune responses resulting from EP-mediated DNA vaccination The EP treatment greatly enhanced the transfection efficiency of the DNA vaccine in the gastrocnemius muscles of the mice (Figure 1A). With a 5 µg intramuscular-injected pHBS dose, the HBsAg expressed in muscles was approximately 2.0 ng at 24 h after injection; with the EP-mediated delivery, approximately 30 ng HBsAg was obtained. The antibody titer in the EP group reached 300 mIU/mL 4 weeks after the vaccination, and remained at high levels (>1000 mIU/mL) for at least 6 months after only 1 immunization (Figure 1B). Cell-mediated immunity was also examined using antigen-specific T cell ELISpot assays. Splenocytes from mice immunized with pHBS/EP responded more readily to HBs epitope stimulation (Figure 1C), compared to the mice immunized by pHBS without EP or treated with EP only.

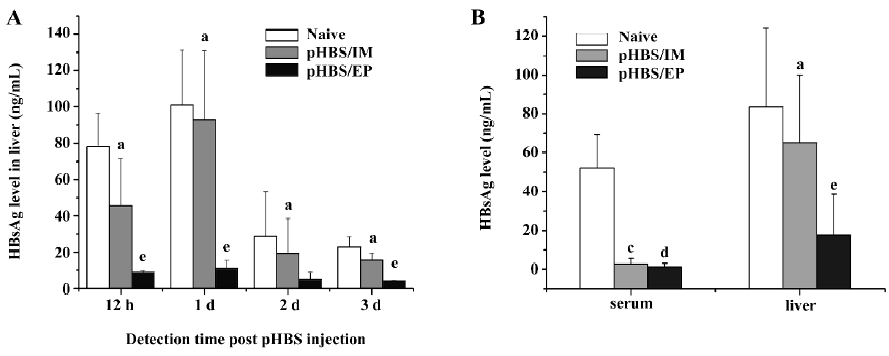
Reduction of HBsAg expression in the liver in immunized mice We used the hydrodynamic injection method to forcefully and effectively transfect the liver cells of the mice to mimic an existing infection model. The hydrodynamic injection method had been widely used to deliver plasmid DNA into hepatocytes by quickly injecting a large volume of solution in a few seconds, in which the gene transfection is based on physical forces only and is unrelated to the vaccination status of the animals. In naive mice, the hydrodynamic injection of 10 µg pHBS resulted in considerable HBsAg expression in the liver within 12 h after the injection, and lasted for about 72 h, but in pHBS/EP-immunized mice with the same dose of pHBS, the HBsAg level in the liver lysates was much lower at all the detection time points. In comparison, the change of HBsAg in the mice injected with the pHBS without EP was similar to naive mice (Figure 2A). We also compared the HBsAg levels in rec-HBs-vaccinated mice and in pHBS/EP-vaccinated mice (Figure 2B). At approximately 4 weeks after vaccination, both groups had high antibody titers. A hydrodynamic injection of pHBS resulted in high circulating HBsAg in the serum in naive mice within 12 h, but in both vaccinated groups, serum HBsAg was almost undetectable due to the neutralization effect of the antibodies. In the liver lysates, the situation was different; the amount of HBsAg in the pHBS/EP-vaccinated group was much lower than that in the protein-vaccinated group.
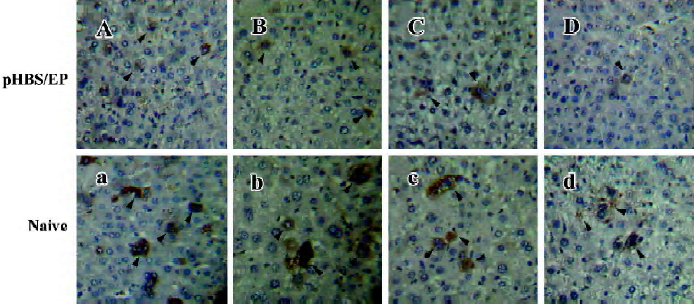
Immunohistological analysis of HBsAg expression in immunized mice After the hydrodynamic injection of pHBS in the mice, the expressed HBsAg in the liver sections was examined by immunohistochemical staining for the HBV surface antigen (Figure 3). In the naive mice, after hydrodynamic injection, HBsAg-positive hepatocytes were easily visible with brown staining throughout the hepatic lobule within 12 h after the injection. Both cytoplasmic and nuclear staining was present. The staining was the heaviest around d 1 after the hydrodynamic injection, and gradually faded from d 2 to 7. This is consistent with the liver HBsAg ELISA measurements described earlier. In comparison, in the pHBS/EP vaccinated mice, the HBsAg-positive hepatocytes were significantly fewer (Table 1) and the color of the stained cells was much lighter.
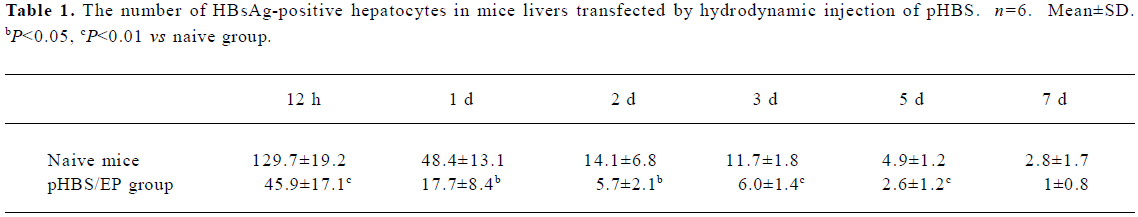
Full table
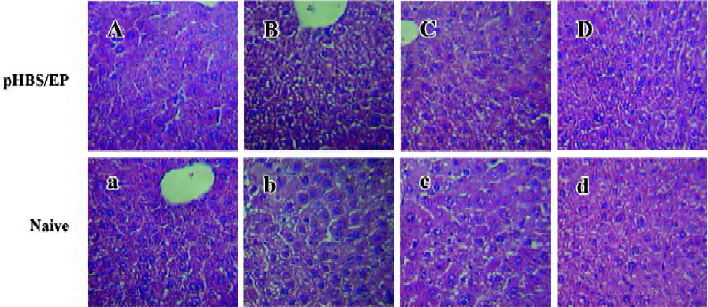
Histological analysis of the liver tissues in immunized mice The histological changes in the liver tissues after the hydrodynamic treatment and during the viral antigen clearance process were examined by HE staining of the liver sections (Figure 4). In naive mice, the hydrodynamic injection procedure did not seem to have caused any lasting tissue damage to the liver. Even in the pHBS/EP-vaccinated animals, the liver cells looked normal. Notably, there was no visible hepatocyte toxicity, tissue inflammation, or even lymphocyte infiltration observed.
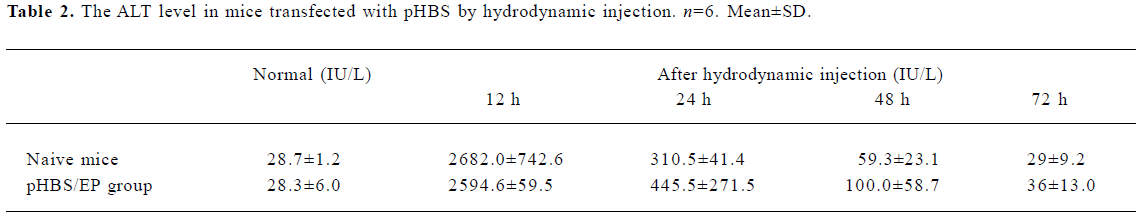
Change of ALT The change of the ALT value was also measured to probe liver tissue toxicity with the hydrodynamic treatment and during the antigen clearance process. The data measured at various time points are listed in Table 2. There was a transient and rapid increase of the serum ALT value immediately after the hydrodynamic injection in both naive and pHBS/EP-vaccinated mice, but they dropped back to close to the normal range within 48 h. There was basically no difference between the naive and the pHBS/EP-vaccinated groups.
Full table
Discussion
DNA vaccination has been regarded as one of the most promising strategies in orchestrating immunological attacks against viral infections for therapeutic purposes. Its ability to elicit cell-mediated immune responses is considered essential for viral clearance and disease eradication. In this study, we showed that DNA vaccination mediated by EP resulted in a strong HBsAg specific antibody and cell-mediated immune responses in mouse models. The results are consistent with several other studies demonstrating the immunopotentiating effect of EP-mediated DNA delivery[12,13].
Specifically for HBV, although recombinant subunit protein vaccines have been generally successful as a prophylactic and are used widely, there are more than 350 million existing virus carriers worldwide who are still waiting for effective treatment. Studies of the mechanisms underlying the immunopathogenesis of HBV infection have suggested that successful resolution of infection was associated with strong T cell-mediated immune responses[20,21]. In particular, CTL that can recognize HBV antigens in hepatocytes and release antiviral cytokines such as interferon-gamma (IFN-γ) were believed to be critical for viral elimination[22,23]. In this regard, many studies adopted the DNA-based vaccination strategy aimed at specifically bringing out cellular-mediated immune responses. To demonstrate the therapeutic potential of these approaches for virus elimination, Oka et al vaccinated the transgenic mice with a high dose of HBV DNA vaccine encoding the HBV envelope protein, and showed that 1 single injection of HBV DNA vaccine resulted in the clearance of serum HBsAg in 28 out of 30 HBV-transgenic mice[15]. Similarly, Pancholi et al showed that DNA-based immunization led to the long-lasting inhibition of HBV replication in a chimpanzee that had been carrying HBV for 12 years. Notably, they reported that the resulting decline of the covalently-closed circular DNA level coincided with the rise of IFN-γ secreting cell numbers, but not CTL[24].
To evaluate whether the strong antibody and cellular responses we observed after EP-mediated DNA vaccination could help to clear existing viral infections in the liver, we developed in this study a pseudo-existing infection model using the hydrodynamic transfection method, which could transfect as much as 40% of hepatocytes[16,17]. Because of its liver specificity and high efficiencies, it is usually used to generate a transient liver-targeted transgenic model for the study of HBV biology and antiviral therapy[25], but unfortunately, the duration of such a transfection is rather short. So in our case, it was impossible for us to vaccinate after the transfection and build up immune responses. We had to pre-immunize the mice to establish the full bloom immune responses before the hydrodynamic transfection, but the hydrodynamic transfection method delivers genes into hepatocytes with physical force, by temporally disrupting the cell membrane and pushing the plasmid DNA in. The pre-established immune responses should not have any effect on the transfection process. So from the view of the immune system, the transfected hepatocytes resembled existing infection, rather than a new infection.
In DNA/EP-immunized mice, the immune system immediately mounted a strong immunological attack to hepatocytes as soon as they started to produce HBsAg. The HBsAg levels detected in the liver were consistently low, compared to those in naive mice, rec-HBs-, or DNA-vaccinated mice. During such a viral antigen clearance process, we observed almost no histological evidence of cell toxicity, suggesting that the antigen clearance is largely due to the down regulation of HBsAg gene expression in liver cells, not cell lysis. This is similar to what Guidotti et al reported in their study after injecting HBsAg-specific CTL clones to HBV transgenic mice[26]. They observed a clearance of HBV mRNA and serum HBsAg, and found that the downregulation of HBsAg was not caused by the cytotoxicity effect of CTL, but rather, by cytokines secreted by the CTL, including IFN-γ. However, in their studies, severe hepatitis was observed, that we did not see based on the ALT measurement. We think it might be due to the short duration of viral antigen expression in transfected hepatocytes by the hydrodynamic injection method.
We acknowledge that the present study does not represent a thorough therapeutic model, as during a normal infection, HBV is able to induce some type of immune suppression that could make the DNA vaccination strategy less effective. Further studies in a real, chronically-infected disease model are necessary in order to understand the complete process of viral clearance after EP-mediated DNA vaccination.
References
- World Health Organization Expert Committee on Biological Standardization. Requirements for hepatitis B vaccine. Bull World Health Organ 1988;66:443-55.
- Buendia MA. Hepatitis B viruses and hepatocellular carcinoma. Adv Cancer Res 1992;59:167-226.
- Maupas P, Goudeau A, Coursaget P, Drucker J, Bagros P. Immunization against Hepatitis B in man. Lancet 1976;1:1367-70.
- Vitiello A, Ishioka G, Grey HM, Rose R, Farness P, LaFond R, et al. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. J Clin Invest 1995;95:341-9.
- Thimme R, Chang KM, Pemberton J, Sette A, Chisari FV. Degenerate immunogenicity of an HLA-A2-restricted hepatitis B virus nucleocapsid cytotoxic T lymphocyte epitope that is also presented by HLA-B51. Virology 2001;75:3984-7.
- Wen YM, Wu XH. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. Lancet 1995;345:1575-6.
- Davis HL. DNA vaccines for prophylactic or therapeutic immunization against hepatitis B virus. Mt Sinai J Med 1999;66:84-90.
- Thermet A, Rollier C, Zoulim F, Trepo C, Cova L. Progress in DNA vaccine for prophylaxis and therapy of hepatitis B. Vaccine 2003;21:659-62.
- Sallberg M, Hughes L, Javadian A, Ronlov G, Hultgren C, Townsend K, et al. Genetic immunization of chimpanzees chronically infected with the hepatitis B virus, using a recombinant retroviral vector encoding the hepatitis B virus core antigen. Hum Gen Ther 1998;9:1719-29.
- Gregoriadis G, Saffie R, de Souza JB. Liposome-mediated DNA vaccination. FEBS Lett 1997;402:107-10.
- Fuller JT, Fuller DH, McCabe D, Haynes JR, Widera G. Immune responses to hepatitis B virus surface and core antigens in mice, monkeys, and pigs after Accell particle-mediated DNA immunization. Ann NY Acad Sci 1995;772:282-4.
- Selby M, Goldbeck C, Pertile T, Walsh R, Ulmer J. Enhancement of DNA vaccine potency by electroporation in vivo. J Biotechnol 2000;83:147-52.
- Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol 2000;164:4635-40.
- Zhao YG, Peng BW, Deng HW, Chen GM, Yang FQ, Shao M, et al. Anti-HBV immune responses in rhesus macaques elicited by electroporation mediated DNA vaccination. Vaccine 2006;24:897-903.
- Oka Y, Akbar SM, Horiike N, Joko K, Onji M. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology 2001;103:90-7.
- Liu F, Song YK, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 1999;6:1258-66.
- Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gen Ther 1999;10:1735-7.
- Tong SP, Li JS, Vitvitski L, Trepo C. Active hepatitis B virus replication in the presence of anti-HBe is associated with viral variants containing an inactive pre-C region. Virology 1990;176:596-603.
- He XQ, Chen GM, Huang Y. Construction and identification of therapeutic double plasmid HBV DNA vaccine. Med J Chin PLA 2003;28:493-6.
- Harty JT, Tvinnereim AR, White DW. CD8 + T cell effector mechanisms in resistance to infection. Annu Rev Immunol 2000;18:275-308.
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T cell differentiation: implications for vaccine development. Nat Rev Immunol 2002;4:251-62.
- Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol 2000;165:1453-62.
- Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 1996;4:25-36.
- Pancholi P, Lee DH, Liu QY, Tackney C, Taylor P, Perkus M, et al. DNA prime/canarypox boost-based immunotherapy of chronic hepatitis B virus infection in a chimpanzee. Hepatology 2001;33:448-54.
- Ketzinel-Gilad M, Zauberman A, Nussbaum O, Shoshany Y, Ben-Moshe O, Pappo O, et al. The use of the hydrodynamic HBV animal model to study HBV biology and anti-viral therapy. Hepatol Res 2006;34:228-37.
- Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, et al. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA 1994;91:3764-8.
