Influence of silybin on biophysical properties of phospholipid bilayers1
Introduction
Silybin (or silibinin) is the main biologically active flavonolignan extracted from the seeds and fruits of milk thistle (Sylibum marianum)[1]. Although the beneficial properties of this plant have been known and used since antiquity, silybin appeared in official medicine as a hepatoprotective substance in the 1970s[2]. Similar to other flavonoids, the range of effects exerted by silybin is very wide[3]. It was found to play the role of anticancer agent against lung[4], prostate[5], ovarian[6] and other cancer types. Silybin derivative dehydrosilybin was found to exert antimalaric effects[7]. Three main mechanisms are supposed to lie in the background of the silybin biological activity: scavenging of free radicals and the chelation of active metal ions, membrane stabilizing function and the influence on RNA synthesis[8]. Recently, silybin was also found to act as a modulator of transporter proteins involved in multidrug resistance of cancer cells: P-glycoprotein[9], multidrug resistance-associated protein 1 (MRP1)[10,11] and breast cancer resistance protein (BCRP)[12,13]. Strong inhibitory effects of silybin on transport carried by MRP1 protein in human erythrocytes was also recorded in our laboratory[14]. Trompier et al[15] have shown that prenylation of dehydrosilybin increases the binding affinity of flavonoid molecule to the MRP1’s nucleotide binding site.
Silybin formulations are commercially often available as complexes of the flavonoid with phospholipids. As shown experimentally, such complexes are characterized by better bioavailability[16] and are more effective free radical scavengers[17,18]. In the murine model, the silybin-containing liposomes composed of phospholipids and cholesterol appeared to be much more effective hepatoprotectors than pure silybin[19].
Despite the fact that the membrane-stabilizing function was suggested as one of the basic molecular mechanisms of silybin activity[8,20] and that silybin-phospholipid complexes are more effective than plain flavonoid, present knowledge about the interactions of silybin with lipid bilayers is very poor. In the study on the influence of silybin on liver microsomal membranes, Parasassi et al[21] suggested that flavonoid molecules were incorporated into the hydrophobic-hydrophilic interface of membranes. Taken into account the antioxidant properties of this compound, it is obvious that it has to interact at least with the polar region of the lipid bilayers. Such interactions seem to be responsible for the protective role of flavonoids against free radicals or active metal ions[22–24]. To elucidate the problem of silybin-phospholipid interactions, we performed this study in which microcalorimetry, fluorescence spectroscopy and electron spin resonance techniques were used. The results of the experiments suggest that silybin interacts with the surface of lipid bilayers and induces small changes in the biophysical properties of lipid membranes. Such an interaction may explain why silybin exerts such beneficial effects without causing any important side effects.
Materials and methods
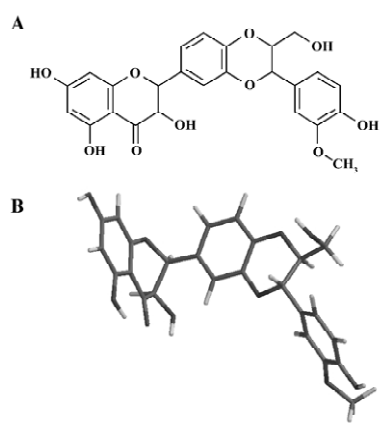
Silybin (its chemical structure is presented in Figure 1A) was purchased from Sigma (St Louis, MO, USA). Laurdan (6-dodecanoyl-2-dimethylaminonaphthalene), Prodan (6-propionyl-2-dimethylaminonaphthalene) and calcein were purchased from Molecular Probes (Eugene, OR, USA). 1,6-diphenyl-1,3,5-hexatriene (DPH) and the lipids used in the experiments, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), egg yolk phosphatidylcholine (EYPC), were from Sigma (St Louis, MO, USA). The spin probes, 5-doxylstearic acid (5-DSA) and 16-doxylstearic acid (16-DSA), were also purchased from Sigma (St. Louis, MO, USA). Tempo-palmitate (TP) was synthesised at the University of Łódź (Łódź, Poland). All other chemicals were of analytical grade.
Small unilamellar liposomes (used in all experiments, except calcein leakage studies) were prepared by sonication of 2 mmol/L phospholipid suspensions in 20 mmol/L Tris-HCl buffer, 0.1 mmol/L EDTA, 50 mmol/L NaCl (pH 7.4) using a UP 200 s sonicator (Dr Hilscher GmBH, Berlin, Germany).
Steady-state fluorescence measurements were performed using a LS 50B spectrofluorimeter (Perkin-Elmer Ltd, Beaconsfield, UK) equipped with a xenon lamp. Fluorescence spectra were collected and processed using FLDM Perkin-Elmer 2000 software (UK).
Theoretical calculations were made using Titan 1.0.8 software (Wavefunction Inc, Irvine, CA, USA and Schrodinger Inc, Portland, OR, USA). Silybin properties were modeled using the AM1 semi-empirical molecular orbital method.
Laurdan and Prodan generalized polarization Laurdan and Prodan generalized polarization (GP) was determined in small unilamellar liposomes made of DPPC, DMPC, and EYPC. Stock solutions of Laurdan (1 mmol/L), Prodan (1 mmol/L) and silybin (5 mmol/L) were prepared in DMSO. Liposomes were incubated with fluorescent dye for 15 min, and afterwards with modulator for 30 min (in dark conditions at room temperature). The final concentrations of substances in the examined samples were: 5 µmol/L Laurdan or Prodan, 200 µmol/L lipid, and 100 µmol/L silybin. The excitation wavelengths for Laurdan ranged from 320 nm to 400 nm (every 10 nm). Prodan fluorescence was excited at 360 nm. Both excitation and emission slit width was 5 nm. The temperature was controlled by a water-circulating bath and measured directly in the cuvette using a platinum thermometer.
Generalized polarization was calculated according to equation 1 given by Parasassi et al[25]. Fluorescence emission intensity of a blue edge of the spectrum (IB) was measured at 440 nm for both fluorescence probes; fluorescence intensity of a red spectrum edge (IR) was recorded at 490 nm for Laurdan and 480 nm for Prodan.
(1)
DPH fluorescence Small unilamellar liposomes were prepared from DPPC, DMPC or EYPC as described earlier. Stock solution of DPH (1 mmol/L) was prepared in tetrahydrofuran. Liposomes were incubated with DPH for 30 min, and afterwards with silybin for the next 20 min (in dark conditions at room temperature). The final concentrations of the compounds in the samples were: 5 µmol/L DPH, 200 µmol/L lipid and 25–150 µmol/L silybin. The experiments were performed at room temperature. DPH fluorescence was excited at 350 nm and its emission was recorded at 428 nm. Fluorescence polarization degree was calculated according to the following equation:
(2)
Where IVV is emission intensity measured with polarizer parallel to the direction of polarization of the excitation light, IVH is the same for the perpendicular polarizer, and G is the instrumental correction factor calculated by FLDM Perkin-Elmer 2000 software (Perkin-Elmer Ltd, Beaconsfield, UK).
DPH fluorescence lifetime measurements were performed with a SLM Aminco 48000S frequency-domain instrument (SLM Instruments Inc, Urbana, Illinois, USA) using a 450 W xenon lamp as a light source, and frequency range of 1–250 MHz. The lifetime of DPH fluorescence decay was calculated by multi-exponential analysis based on phase shifts and demodulation parameters, using fluorimeter software. As DPH fluorescence decay was not a single exponential function, the average lifetime <τ> was calculated according to the following equation:
(3)
Where τi is fluorescence lifetime, and fi is fractional intensity contribution.
Differential scanning calorimetry (DSC) For each sample, 2 mg of lipid was dissolved in an appropriate amount of silybin stock solution (5 mmol/L, in ethanol) to obtain the desired drug:lipid molar ratio. We studied silybin:lipid mixtures at 0.03, 0.045 and 0.06 molar ratios because in these concentrations in the experiments with other flavonoids, we observed pronounced changes in thermal behavior of lipids, but bilayers were not perturbed by flavonoids as much[26]. The samples were dried under the stream of nitrogen and placed under vacuum for at least 2 h. Then 15 µL of 20 mmol/L Tris-HCl buffer (150 mmol/L NaCl, 0.5 mmol/L EDTA, pH 7.4) was added to each sample. Hydrated mixtures were heated to the temperature circa about 10 °C higher than main phase transition temperature of a given lipid, and vortexed until a homogeneous dispersion was obtained. Samples were sealed in aluminum pans and scanned at the rate of 1.25 °C/min. Measurements were performed using a Rigaku calorimeter (Rigaku, Tokyo, Japan), which was partially rebuilt in our laboratory. Samples were scanned immediately after preparation. For each drug:lipid molar ratio, at least 2 samples were prepared; each sample was scanned at least 4 times. Calorimetric data were collected and processed offline using software developed in our laboratory.
Electron spin resonance spectroscopy (ESR) Samples for the ESR experiments were prepared from ethanol solutions similar as those for DSC. Dry lipid films formed from DPPC, DMPC or EYPC (5 mg per sample) and silybin were hydrated with the addition of 250 µL of Tris-HCl buffer (20 mmol/L, 150 mmol/L NaCl, 0.5 mmol/L EDTA, pH 7.4). Then the samples were shaken at room temperature for 5 min to obtain multilamellar liposomes. All samples were incubated for at least 12 h at 4 °C before the experiment.
All spin probes (TP, 5-DSA and 16-DSA) were dissolved in ethanol (10 mmol/L stock solutions). The lipid:spin probe molar ratio was 0.015 for DMPC and EYPC, and 0.05 for DPPC. An appropriate amount of spin probe stock solution was dried on the glass tube walls. Afterwards, the suspension of liposomes was added to the tube and the mixture was mechanically shaken. After 20 min, the spin probes were incorporated into lipid bilayers and the liposomes were sufficiently labeled for the ESR experiment.
The labeled liposomes were then transferred into a glass capillary (1 mm inner diameter). All spectra were recorded at 25 °C using a standard SE/X-28 electron spin resonance spectrometer (Wroclaw Technical University, Wroclaw, Poland) operating in the X-band. In order to estimate the mobility of the spin probes TP and 16-DSA during their isotropic weakly-restricted rotational motion, the tumbling correlation time (τc) was calculated using Kivelson’s method[27]:
τC=6.5×10-10w0 [(h0/h-1)0.5–1] (4)
where w0, h0 and h-1, are parameters taken from the ESR spectrum, w0 is the midfield line width, and h0, h-1 are mid- and high-field line amplitudes.
The 5-DSA spin probe under experimental conditions had strongly restricted motion in the membrane system. In this case, the degree of restriction of its motion was expressed by the order parameter (S), which was a measure of the relative fluidity in the membranes. The order parameter was calculated from the following equation:
S=[(A|| -A⊥)/(Azz–Axx)](a/a’) (5)
Where A|| and A⊥ are the maximal and minimal hyperfine splitting constants measured, respectively, Azz and Axx are the hyperfine splitting tensors measured for probes in a crystal matrix, and a and a’ are the isotropic hyperfine splitting constants for nitroxides in the crystal matrix:
a=1/3 (Azz+2Axx) (6)
And in the membranes:
a’ = 1/3 (A|| +2A⊥) (7)
Calcein leakage studies Unilamellar EYPC liposomes were prepared by the extrusion method. First, the samples containing chloroform solution of lipid were evaporated under a stream of nitrogen. The remnants of organic solvent were removed under vacuum for at least 2 h. Then the lipid film was hydrated by vortexing with 1 mL of 36 mmol/L solution of calcein in 10 mmol/L HEPES buffer (pH 7.4). Liposomal suspensions were extruded through polycarbonate filters, 400 nm and subsequently 100 nm, using a high pressure extruder (PPH Marker, Wroclaw, Poland). The calcein-containing vesicles were separated from the free calcein by molecular filtration on a Sepharose 4B column eluted with 10 mmol/L HEPES buffer,150 mmol/L NaCl; 1 mmol/L EDTA (pH 7.4). The final lipid concentration in the liposome suspension was 200 µmol/L. The liposomes containing calcein were incubated with silybin (4–84 µmol/L) for 5 min (in dark conditions at room temperature). Longer incubation times did not change the results. The degree of calcein release was determined spectrofluorimetrically. The fluorescence was excited at 490 nm and emission was recorded at 520 nm. Both the excitation and emission slit widths were set at 4 nm. The results of the experiments are presented as the percentage of released calcein (Frelease), calculated according to the following equation:
(8)
Where Ft is the fluorescence intensity of liposomes after the addition of silybin, F0 is the fluorescence intensity of liposomes, and F∞ is the total (100%) fluorescence intensity measured after lysis of liposomes with Triton X-100 (detergent final concentration 0.5%).
Results
Molecular modeling of silybin properties Molecular modeling showed that the silybin molecule was not planar and had 2 bends between the policyclic structures (Figure 1B). These bends are likely to be responsible for the flexibility of the silybin molecule. The octanol:water partition coefficient of silybin was also calculated (lg P=1.53).
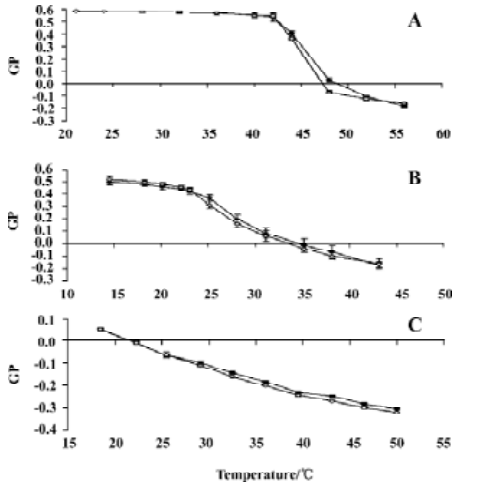
Laurdan and Prodan generalized polarization The fluorescent probe Laurdan was employed to study the influence of silybin on the thermal behavior of liposome membranes formed from various lipids. For DPPC and DMPC liposomes, an abrupt change of the GP value corresponded to the phospholipid main phase transition. As shown in Figure 2, silybin did not influence any of the studied phosphatidylcholine bilayers significantly in the studied temperature range. Transition temperatures of DPPC and DMPC were almost unchanged in the presence of the flavonoid. Only in case of DMPC did silybin cause a slight increase of GP values in the gel state of the lipid (Figure 2B). The addition of 100 μmol/L silybin to the EYPC model membranes resulted in the slight decrease of Laurdan GP values in temperatures higher than 30 °C (Figure 2C).
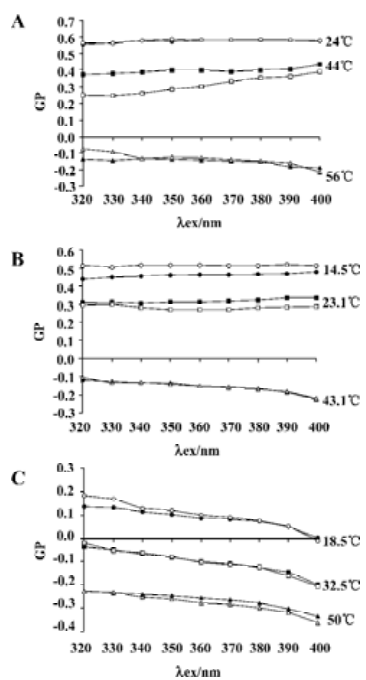
The shapes of the Laurdan fluorescence spectra depended on the lipid phase state, showing an emission maximum near 440 nm in the gel state and near 490 nm in the liquid-crystalline state. Measuring Laurdan generalized polarization as a function of excitation wavelength allows not only characterization of the phase state of the lipid bilayer, but also detects the phase separation, that is, the existence of lipid microdomains[25]. The dependence of Laurdan GP on the excitation wavelength recorded for DPPC, DMPC and EYPC is shown in Figure 3. For DPPC (Figure 3A) and DMPC (Figure 3B), the GP (λex) functions were plotted for the temperatures below, above and during the main phase transition. For pure lipids, the GP was not dependent on excitation wavelength in temperatures below the phase transition. The curve representing GP versus the λex relationship ascended during the phase transition and descended in temperatures above the lipid melting temperature. The addition of silybin did not change either the DPPC or DMPC bilayer properties significantly. EYPC bilayers were in a liquid-crystalline state in all the temperatures studied; that is why the negative slope of GP on λex dependence was observed (Figure 3C); the addition of silybin did not influence the EYPC bilayers.
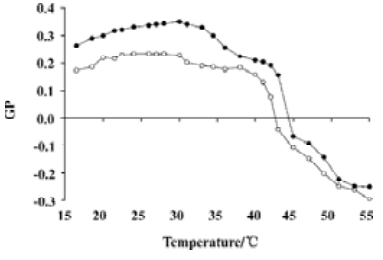
Prodan was additionally used to investigate the effect of silybin on DPPC membranes as this probe allowed the monitoring of not only the main phase transition, but also the phospholipid pretransition. As shown in Figure 4, the addition of 100 μmol/L silybin to the DPPC liposomes caused the decrease of Prodan GP values. This decrease was more pronounced in temperatures in which DPPC was in gel state. The biggest difference between the curve obtained for pure lipid and for the DPPC:silybin mixture could be observed in the region between 30 and 35°C, which suggested that silybin strongly influenced DPPC pretransition.
DPH fluorescence DPH, a fluorescent probe localizing deeply in the bilayer core[28], was employed to study the influence of silybin on the hydrophobic region of model membranes. The recorded values of DPH fluorescence polarization were dependent on the type of phospholipid. The values were near 0.4 in the DPPC bilayers that were in the gel phase at the temperature of the experiment, 0.2 in DMPC that was near the phase transition, and 0.1 in the EYPC liquid-crystalline model systems. The addition of silybin had almost no effect on DPH fluorescence polarization in the DPPC membranes, whereas it caused considerable increase in DPH polarization values in DMPC (about 17% at 100 μmol/L of silybin) and in the EYPC bilayers (near 30% at 100 μmol/L). No significant influence of the solvent used (DMSO) on DPH fluorescence polarization was recorded.
At the same time, quenching of DPH fluorescence by silybin was observed. Stern-Volmer plots of DPH quenching were linear (Figure 5). The extent of quenching was similar in all lipids studied.
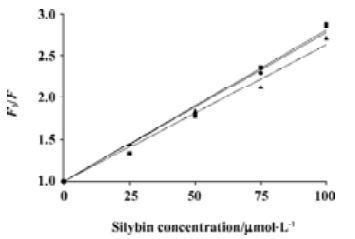
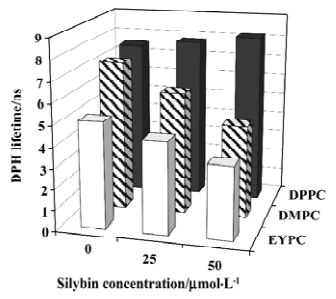
As silybin-induced DPH fluorescence polarization increase is accompanied by the decrease of fluorescence intensity, we decided to study the DPH fluorescence lifetimes too. The values of the DPH fluorescence lifetime in 3 investigated lipids and in their mixtures with silybin are shown in Figure 6. In pure lipids, DPH had the longest lifetime in membranes composed of DPPC, and the shortest in the EYPC bilayers. The addition of silybin into DPPC liposomes caused no significant changes in the DPH lifetime. Contrary to DPPC, in the liquid-crystalline DMPC and EYPC membranes, the shortening of the DPH fluorescence lifetime was observed when the concentration of silybin was raised.
Differential scanning calorimetry To study the influence of silybin on the thermotropic properties of lipid bilayers, microcalorimetry was employed. The changes of transition parameters of DMPC and DPPC caused by the addition of the drug are presented in Table 1. In pure lipids, both pre-transition and gel-liquid crystalline transition were recorded. Pretransition vanished in both the DMPC and DPPC model membranes, even at the lowest silybin:lipid molar ratio used (0.03). Incorporation of silybin into the DMPC model systems produced the lowering of lipid main phase transition temperatures accompanied by calorimetric peak broadening. Transition enthalpy of DMPC decreased in the presence of silybin, however, no clear dependence of this parameter on drug concentration was observed. DPPC membranes were influenced by silybin to a lesser extent than those of DMPC. First, we observed that the first thermogram recorded immediately after the sample preparation always looked different than the following ones. Different calorimetric peak shape and a stronger lowering of the transition temperature were observed during the first scan. The transition parameters for DPPC presented in Table 1 were calculated only for thermograms recorded after the samples reached equilibrium. The transition temperature of the DPPC bilayers were slightly lowered in the presence of silybin, whereas no significant changes of transition enthalpy were observed. Both parameters did not show clear dependence on silybin concentra-tion.
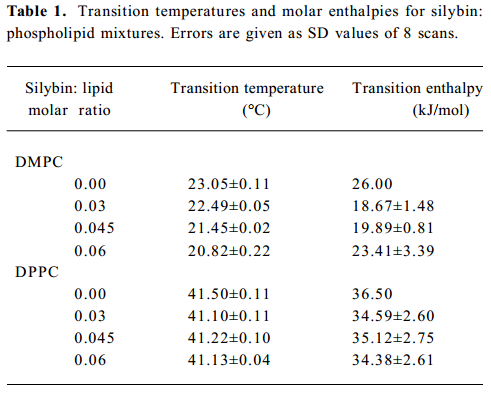
Full table
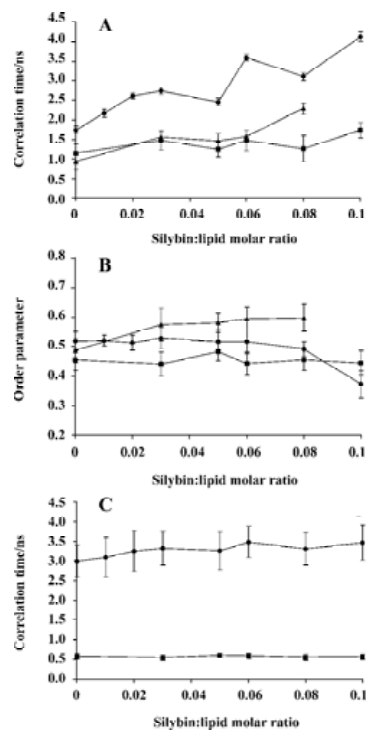
Electron spin resonance spectroscopy Three types of spin probes used in the ESR experiments (TP, 5-DSA and 16-DSA) enabled us to follow the mobility changes induced by silybin in different regions of phosphatidylcholine bilayers. Tempo-palmitate was used to monitor the lipid polar heads region of the model membrane, 5-DSA probed polar/apolar interface, while 16-DSA delivered information from the hydrophobic region close to the bilayer centre. The spectral parameters obtained for the spin probes are presented in Figure 7. The results showed that the addition of silybin to liposomes caused TP spin probe immobilization in all the studied lipids (Figure 7A). On the other hand, silybin exerted almost no effect on order parameter of the 5-DSA spin probe (Figure 7B), only in case of DMPC bilayers slight immobilization of 5-DSA was visible. The addition of silybin had no effect also on spin probe 16-DSA located deeply in the membrane core (Figure 7C). It was also noticed that the effect of silybin on model membranes was not dependent on the bilayer phase state. Although all the experiments were performed at 25 °C, the temperature in which DPPC was in the gel state, DMPC was slightly above main phase transi-tion, and EYPC was in the liquid-crystalline state, the influence of silybin on the model membranes composed of different lipids was similar, no matter which spin probe was employed.
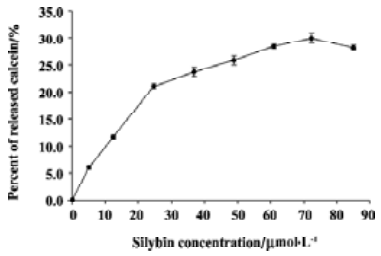
Calcein leakage studies The influence of silybin on the EYPC bilayer structure integrity and stability was investigated using calcein leakage assay. Calcein was entrapped in liposomes in such a concentration that its fluorescence was self-quenched. Disturbance of lipid bilayer by silybin caused the increase of membrane permeability and concentration-dependent release of the fluorescence probe from liposomes (Figure 8). The release of the fluorescence probe grew when the concentrations of silybin were raised to 25 μmol/L, whereas for higher flavonoid concentrations, only a slight further increase of calcein leakage was observed. Maximal release of calcein caused by the flavonoid was about 29%. It was observed that DMSO alone was able to cause only slight calcein leakage (up to 5%).
Discussion
In the present work, we have systematically studied the influence of silybin on biophysical properties of phospholipid bilayers. In our experiments, we used model membranes composed of zwitterionic lipid, phosphatidylcholine. Two phosphatidylcholine species differing in length of the acyl chains were used (DPPC and DMPC), as well as a mixture of natural phosphatidylcholines (EYPC).
The value of the octanol/water partition coefficient calculated for silybin indicated relatively low hydrophobicity of this compound. Based on this feature, one should not expect strong silybin–membrane interactions. On the other hand, molecular modeling showed that the silybin molecule was not planar, and high flexibility of its molecule could be expected. The ability of the silybin molecule to adopt different conformations is likely to influence its interaction with phospholipid bilayers. The importance of spatial conformation of flavonoid molecules for their intercalation into model membranes has been previously suggested[29,30].
To further characterize the interactions of silybin with phospholipid bilayers, fluorescence spectroscopy was em-ployed. Laurdan is a fluorescent probe possessing lauric acid tail that anchors in the hydrophobic core of the membrane, and in which fluorophore resides at the level of the phospholipid glycerol backbone. The spectral properties of this probe are extremely dependent on the amount of the water molecules penetrating into the hydrophobic-hydrophilic interface of the membrane and on their dynamics, especially on the dipolar relaxation process in Laurdan’s environment[31]. Since gel and liquid-crystalline phases of phospholipid bilayers differ in hydration and water dynamics, Laurdan constitutes a useful tool to study lipid thermotropic behavior. As observed by Laurdan fluore-scence, the addition of silybin into the phosphatidylcholine bilayer did not cause any pronounced effects. GP values for pure lipids and the lipid:silybin mixtures were virtually the same, only DMPC in gel state, and EYPC in high temperatures could small influences of silybin addition be observed. The influence of this compound on the temperature of the main phase transition of DMPC and DPPC was also negligible.
Laurdan can also be used to detect the phase separation, that is, the existence of lipid microdomains[25]. Measuring Laurdan GP as a function of excitation wavelength allowed us to check whether the addition of silybin to the model bilayers affected lateral membrane organization. In no case such an influence of silybin was detected. In general, the presence of silybin in phospholipid systems seemed not to alter significantly the packing of the lipid membrane in the vicinity of Laurdan molecules.
Prodan possesses the same fluorophore as Laurdan, but connected to a shorter propionyl tail. That is why it is more loosely anchored to the lipid bilayer and located closer to the membrane surface than Laurdan[31]. By following Prodan’s GP as a function of temperature, the pretransition of the lipid bilayer can be detected. The biggest difference of the course of GP on temperature dependence recorded in the DPPC:silybin mixture as compared to pure lipid was visible in temperatures typical for DPPC pretransition. This indicates that silybin interacts with the polar head-group region of the DPPC membrane strongly enough to affect pretransition. In contrast to Laurdan, Prodan exhibits quite significant fluorescence in water, which influenced the values of GP calculated for this probe. The possibility of interaction between Prodan and silybin present in the buffer was excluded by the experiment in which we observed no apparent influence of flavonoid on the emission spectrum of the fluorescent probe. It was shown that the Prodan partition coefficient between the phosphatidylcholine bilayer and water strongly depended on the lipid’s phase state, being higher in liquid-crystalline phase[32]. Lower GP values were observed for the silybin:DPPC mixture than for pure lipid, which could be at least partially caused by the higher number of Prodan molecules residing outside the membrane in the presence of the flavonoid. Fluorescence originating from water-immersed Prodan molecules raised the total emission intensity of a red spectrum edge thus reducing the GP value. We hypothesized that the presence of silybin decreased Prodan partitioning into DPPC bilayers, especially in the gel phase. Such an effect is likely to be caused by silybin interaction with the same membrane region where Prodan is located.
Fluorescence of DPH, the probe localizing in the hydrophobic core of the membrane, was also affected by the addition of silybin to the liposomes. The increase of fluorescence polarization was observed in DMPC and EYPC bilayers, but not in those of DPPC. However, this increase was likely to be apparent as the presence of silybin resulted in DPH fluorescence lifetime shortening in these model systems where the polarization increase was recorded. Previous researchers[21] reported only a slight influence of silybin on DPH fluorescence anisotropy when incorporated into rabbit liver microsomes. We have also observed that the addition of silybin into liposomes caused a reduction in DPH fluorescence intensity. Other flavonoids have been also reported to be able to quench DPH fluorescence[33]. As Stern-Volmer plots of fluorescence quenching were linear, and lifetime shortening was seen in EYPC and DMPC model membranes, we concluded that silybin-induced changes of DPH fluorescence were the manifestation of collisional quenching of this probe by the flavonoid. Where such a quenching mechanism seemed probable in EYPC and DMPC bilayers, static quenching seemed to prevail in more compactly-packed DPPC bilayers in which no lifetime shortening was observed. The difference in quenching mechanisms between different phosphatidylcholine species was likely to result from loosely-packed structures of liquid-crystalline bilayers of EYPC or DMPC, and more compact structures of DPPC being in gel state in the temperature of the experiment. DPH fluorescence lifetime shortening observed in EYPC and DMPC in the presence of silybin could accompany the quenching, but the DPH lifetime was also reported to be shortened by increased water penetration into the membrane interior[34]. In our opinion, this was not the case since fluorescence of Laurdan was not altered by the flavonoid. It is doubtful that silybin would have caused distinct hydration increase deep in the membrane core without even slightly changing the amount of water associated with the hydrophobic–hydrophilic interface of membranes.
We assumed the quenching of DPH fluorescence by silybin was caused by the interaction of the 2 molecules inside the membrane, requiring direct contact between the 2 entities. Quenching by FRET (fluorescence resonance energy transfer) was excluded as the absorption spectrum of the flavonoid did not overlap with the emission spectrum of DPH. Silybin’s molecule is quite long, and when incorporated into the lipid bilayer, it could penetrate deeply into the membrane. It has been shown previously that flavonoids are able to influence the fluorescence of anthroyloxy-fatty acids with fluorophores located at different depths from the membrane surface[35,23]. It has also been demonstrated that the membrane distribution of flavonoids is quite broad[36] which suggests that at least a portion of silybin molecules could interact with DPH when incorporated into the lipid bilayer.
Microcalorimetry was employed to investigate the influence of silybin on the thermotropic properties of the DMPC and DPPC bilayers. It has been demonstrated that the flavonoid altered the structure of the phospholipid bilayer in the gel state as the addition of the drug resulted in the vanishing of pretransition in both lipids used. The disappearance of DPPC pretransition recorded by DSC was in accordance with silybin-induced changes in Prodan-generalized polarization observed by fluorescence spectroscopy. Studying the influence of silybin on main lipid phase transition, we noticed that the flavonoid affected mainly the transition temperature, while its impact on the transition enthalpy was weaker. Such behavior is recognized to be characteristic for modifiers that interact mainly with the polar head group region of the bilayer and only partially penetrate into the hydrocarbon region[37]. Similar changes on the DPPC thermotropic properties as exerted by silybin have been previously observed for other flavonoids[29]. Silybin perturbed the DMPC bilayer structure stronger than the DPPC model membranes. In our opinion, this could be explained by the fact that DMPC, possessing shorter acyl chains than DPPC, is also characterized by weaker interactions between hydrocarbon chains and more loosely-packed structures which could facilitate silybin incorporation into DMPC membranes. It has been also observed that the shapes of calorimetric peaks of the DPPC:silybin mixtures varied as a function of time as if the mixture reached equilibrium only after some time after the sample preparation. Quercetin and hesperetin have been also demonstrated to exhibit time-dependent changes in thermograms recorded in DPPC membranes[29].
ESR experiments showed that silybin did not change the mobility of spin probes embedded either in polar/apolar interface or in hydrophobic regions of the membrane. On the other hand, the tumbling correlation time of Tempo-palmitate was increased in the presence of silybin which indicated the immobilization of the spin probe. Based on the results of the ESR experiments, we concluded that silybin interacted with the polar head group region of the lipid bilayers while exerting almost no effect on deeper regions of the membrane. Similar conclusions were drawn previously in the ESR study on quercetin influence on human erythrocyte membranes[38].
We have also shown that silybin was able to increase the permeability of liquid crystalline EYPC membranes to calcein. The overall increase of EYPC membrane permeability in the presence of silybin was not very large. The increase was most rapid in low silybin concentrations, whereas higher flavonoid concentrations induced only slight further permeability increase, as if the effect exerted by this compound on the membrane became saturated. It seemed likely that the introduction of low amounts of silybin molecules into the membrane resulted in the transient changes of bilayer packing and the appearance of membrane defects. Flavonoids possessing several hydroxyl groups in the molecule were postulated to form multiple hydrogen bonds with polar heads of phospholipids. Such a mechanism was proposed to explain the protective properties of flavonoids against membrane oxidation and solubilization by detergents[23,24]. Therefore, it seemed possible that increasing amounts of silybin molecules formed a network stabilizing the surface of liposome membrane. At concentrations high enough, the silybin-induced processes of membrane destabilisation and sealing balanced, thus leading to the saturation of calcein leakage. It should be also noted that EYPC membranes are more loosely packed and more vulnerable to perturbation than natural membranes. We observed that silybin in concentrations up to 150 μmol/L was not able to induce erythrocyte hemolysis (data not presented). In our opinion, silybin is not likely to induce strong alterations of natural membrane integrity and stability.
The results presented show that silybin interacts mainly with the polar head group region of the lipid bilayers. The ability of silybin to quench the fluorescence of DPH molecules in the membranes indicate that at least some of the flavonoid molecules can partition into the hydrophobic region of the membrane. This partitioning, however, does not change the biophysical properties of deeper membrane regions in a significant way. In our opinion, such a behavior of silybin in membranes is in accordance with its postulated biological functions, such as membrane stabilization and cell protection. Moreover, the absence of serious perturbations of membrane biophysical properties induced by silybin may correspond to the observed lack of serious side-effects of this drug, in spite of its use in therapy in relatively high doses. Further research is needed, however, to determine the precise membrane localization of silybin molecules.
References
- Crocenzi FA, Roma MG. Silymarin as a new hepatoprotective agent in experimental cholestasis: new possibilities for an ancient medication. Curr Med Chem 2006;13:1055-74.
- Giese LA. Milk thistle and the treatment of hepatitis. Gastroen-terol Nurs 2001;24:95-7.
- Hendrich AB. Flavonoid-membrane interactions: possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol Sin 2006;27:27-40.
- Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res 2003;23:2649-55.
- Singh RP, Agarwal R. Prostate cancer prevention by silibinin. Curr Cancer Drug Targets 2004;4:1-11.
- Gallo D, Giacomelli S, Ferlini C, Raspaglio G, Apollonio P, Prislei S, et al. Antitumour activity of the silybin-phosphatidylcholine complex, IdB 1016, against human ovarian cancer. Eur J Cancer 2003;39:2403-10.
- de Monbrison F, Maitrejean M, Latour C, Bugnazet F, Peyron F, Barron D, et al. In vitro antimalarial activity of flavonoid derivatives dehydrosilybin and 8-(1;1)-DMA-kaempferide. Acta Trop 2006;97:102-7.
- Valenzuela A, Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res 1994;27:105-12.
- Maitrejean M, Comte G, Barron D, El Kirat K, Conseil G, Di Pietro A. The flavanolignan silybin and its hemisynthetic deriva-tives, a novel series of potential modulators of P-glycoprotein. Bioorg Med Chem Lett 2000;10:157-60.
- Nguyen H, Zhang S, Morris ME. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J Pharm Sci 2003;92:250-7.
- Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J 2005;272:4725-40.
- Cooray HC. Janvilisri, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun 2004;317:269-75.
- Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res 2004;21:1263-73.
- Lania-Pietrzak B, Michalak K, Hendrich AB, Mosiadz D, Grynkiewicz G, Motohashi N, et al. Modulation of MRP1 protein transport by plant, and synthetically modified flavonoids. Life Sci 2005;77:1879-91.
- Trompier D, Baubichon-Cortay H, Chang XB, Maitrejean M, Barron D, Riordon JR, et al. Multiple flavonoid-binding sites within multidrug resistance protein MRP1. Cell Mol Life Sci 2003;60:2164-77.
- Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur J Drug Metab Pharmacokinet 1990;15:333-8.
- Carini R, Comoglio A, Albano E, Poli G. Lipid peroxidation and irreversible damage in the rat hepatocyte model. Protection by the silybin-phospholipid complex IdB 1016. Biochem Pharmacol 1992;43:2111-5.
- Comoglio A, Tomasi A, Malandrino S, Poli G, Albano E. Scavenging effect of silipide, a new silybin-phospholipid complex, on ethanol-derived free radicals. Biochem Pharmacol 1995;50:1313-6.
- Maheshwari H, Agarwal R, Patil C, Katare OP. Preparation and pharmacological evaluation of silibinin liposomes. Arzneimittel-forschung 2003;53:420-7.
- Letteron P, Labbe G, Degott C, Berson A, Fromenty B, Delaforge M, et al. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice. Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem Pharmacol 1990;39:2027-34.
- Parasassi T, Martellucci A, Conti F, Messina B. Drug-membrane interactions: silymarin, silibyn and microsomal membranes. Cell Biochem Funct 1984;2:85-8.
- Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavanoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med 1995;19:481-6.
- Erlejman AG, Verstraeten SV, Fraga CG, Oteiza PI. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res 2004;38:1311-20.
- Oteiza PI, Erlejman AG, Verstraeten SV, Keen CL, Fraga CG. Flavonoid-membrane interactions: a protective role of flavonoids at the membrane surface? Clin Dev Immunol 2005;12:19-25.
- Parasassi T, De Stasio G, d’Ubaldo A, Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys J 1990;57:1179-86.
- Hendrich AB, Malon R, Poła A, Shirataki Y, Motohashi N, Michalak K. Differential interaction of Sophora isoflavonoids with lipid bilayers. Eur J Pharm Sci 2002;16:201-8.
- Kocherginsky N, Swartz HM. Chemical reactivity of nitroxides in nitroxide spin labels. Boca Raton, FL: CRC Press; 1995.
- Kaiser RD, London E. Location of diphenylhexatriene (DPH) and its derivatives within membranes: comparison of different fluorescence quenching analyses of membrane depth. Biochemistry 1998;37:8180-90.
- Saija A, Bonina F, Trombetta D, Tomaino A, Montnegro L, Smeriglio P, et al. Flavonoid-biomembrane interactions: a calorimetric study on dipalmitoylphosphatidylcholine vesicles. Int J Pharm 1995;124:1-8.
- van Dijk C, Driessen AJ, Recourt K. The uncoupling efficiency and affinity of flavonoids for vesicles. Biochem Pharmacol 2000;60:1593-600.
- Parasassi T, Krasnowska EK, Bagatolli L, Gratton E. Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J Fluorescence 1998;8:365-73.
- Krasnowska EK, Gratton E, Parasassi T. Prodan as membrane surface fluorescence probe: partitioning between water and phospholipid phases. Biophys J 1998;74:1984-93.
- Schoefer L, Braune A, Blaut M. A fluorescence quenching test for the detection of flavonoid transformation. FEMS Microbiol Lett 2001;204:277-80.
- Stubbs CD, Ho C, Slater SJ. Fluorescence techniques for probing water penetration into lipid bilayers. J Fluorescence 1995;5:19-28.
- Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys 2000;373:102-9.
- Scheidt HA, Pampel A, Nissler L, Gebhardt R, Huster D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectro-scopy. Biochim Biophys Acta 2004; 1663: 97–107.
- Jain MK, Wu NM. Effect of small molecules on the dipalmitoyl lecithin liposomal bilayer: III. Phase transition in lipid bilayer. J Membr Biol 1977;34:157-201.
- Pawlikowska-Pawlega B, Gruszecki WI, Misiak LE, Gawron A. The study of the quercetin action on human erythrocyte mem-branes. Biochem Pharmacol 2003;66:605-12.
