Drug brain distribution following intranasal administration of Huperzine A in situ gel in rats1
Introduction
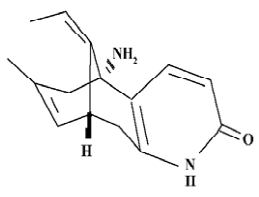
Huperzine A (Hup A), extracted from a club moss (Huperzia serrata), is an unsaturated sesquiterpene alkaloid with a pyridone moiety and primary amino group. Its empirical formula is C15H18N2O, and its molecular weight is 242. Chemically, Hup A is 9-amino-13-ethylidene-11-methyl-4-azatricyclo [7.3.1.0(3.8)] trideca-3(8), 6, 11-trien-5-one, and its structure is shown in Figure 1. Hup A is a powerful and reversible inhibitor of acetyl cholinesterase. The agent easily penetrates the blood brain barrier (BBB) and it is a promising therapeutic agent for Alzheimer's disease. There are several forms of Hup A, including tablet, capsule, transdermal delivery system, injection and sustained release injectable microsphere. Because Hup A can influence the cholinergic system and results in side effects to peripheral tissues, it is important to improve Hup A brain-targeting efficiency by targeting routes[1].
Intranasal drug administration offers rapid absorption to the systemic blood avoiding first-pass metabolism, and it has been shown to present a safe and acceptable alternative to the parenteral administration of a lot of drugs. Several studies have shown a direct route of transport from the olfactory region to the central nervous system in animal models, without prior absorption to the circulating blood[2].
Gel formulations can increase the contact time with the mucosa and thereby facilitate the uptake extent of the drug. On the other hand, in order to target the drug to the olfactory mucosa, higher deposition of the drug in the nasal area is crucial, so low viscosity of the formulations is required. In situ gel was designed to meet the requirement of the above purposes. In this study, in situ gel of gellan gum was used. In an ion-free environment, the solution of gellan gum exhibits a low viscosity, which forms a strong gel at physiological cation concentration. It is liquid-like in vitro and can be administered easily as a drop or by a spray device and become semi-solid as soon as there is contact with the mucosa.
The aim of this paper was to study the drug brain distribution in the rats following unilateral intranasal administration of the Hup A in situ gel. Intravenous administration of Hup A injection and administration of the oral formulation (Hup A tablets dispersed in distilled water) were compared with intranasal administration.
Materials and methods
Chemicals Hup A in situ gel (2 g/L) was obtained from the Division of Pharmaceutics of Shanghai Institute of Pharmaceutical Industry (Shanghai, China). The Hup A injection (0.2 g/L) was purchased from Zhejiang Wanbang Pharmaceutical Limited Cooperation (Taizhou, Zhejiang, China). The oral formulation (0.15 g/L) was made by dispersing Hup A tablets in distilled water. Hup A tablets (50 µg per tablet) were produced by Shanghai Fudan Fuhua Pharmaceutical Limited Cooperation (Shanghai, China). HPLC-grade methanol was purchased from N
Animals Male Sprague-Dawley rats weighing about 300 g were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China).
For the intranasal administration, the rats were anesthetized with an ip injection of 10% chloral hydrate solution, and 25 µL of the nasal formulation (Hup A in situ gel) was administered via a PE 10 tube attached to a microlitre syringe inserted 1 cm into left nostril of rats at a 166.7 µg/kg dose. For the iv administration, the Hup A injection was delivered (166.7 µg/kg) through the caudal vein. Oral gavage of Hup A (500 µg/kg) was performed by attaching a stainless steel feeding needle to a 1 mL syringe containing the oral formulation. At 0.08, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4 and 6 h after the intranasal or oral dose, and at 0.03, 0.08, 0.25, 0.5, 1, 1.5, 2, 3, 4, and 6 h after the iv dose, the animals were decapitated and the blood was collected from the trunk. Then the skull was cut open and the olfactory bulb, hippocampus, cerebrum and cerebellum were carefully excised. The brain tissues were quickly rinsed with saline and blotted with filter paper to remove the blood taint and macroscopic blood vessels as much as possible. After weighing, the olfactory bulb, hippocampus, cerebrum and cerebellum samples were homogenized with 0.4, 0.4, 0.5, and 1 mL water in tissue homogenizers, respectively. Blood samples were anticoagulated with heparin and centrifuged at 5000 ×g for 10 min to obtain the plasma.
Both plasma and brain tissue homogenates were stored in a deep freezer at -20 °C until HPLC analysis. Measurements were repeated on 4 rats at each time point.
Sample preparation For the 175 µL plasma samples or 0.2 g brain tissue homogenates, 10 µL IS methanol solution (2 mg /L for the plasma sample and 1 mg/L for the brain tissue sample), 100 µL NaCO3-Na2BO4 buffer (pH 11.5) and 2 mL chloroform were added. The mixture was vortexed for 5 min and centrifuged at 5000 ×g for 10 min. Then the organic phase was transferred to a conical tube and evaporated to dryness under a gentle fluid of nitrogen at 40 °C. For the plasma sample, the residue was reconstituted in the 50 µL mobile phase, and then 20 µL supernatant was injected onto the HPLC system. For the brain tissue samples, the residue was reconstituted in 100 µL 0.01 mol/L acetic acid solution, and then 40 µL supernatant was injected onto the HPLC system after centrifugation at 20 000 ×g for 5 min. Samples were quantified using peak area ratio of Hup A to IS.
High performance liquid chromatography The HPLC system consisted of the LC-10AD VP delivery system, RF-10AXL fluorescence spectrophotometric detector, and CLASS-VP chromatographic integrator (Shimadzu, Japan). The separation was performed on a Kromasil C-8 column (5 µm×4.6 mm×15 mm). The mobile phase consisted of methanol:water:triethanol amine (45:55:0.05). Briefly, a flow rate of 1 mL/min, running time of 12 min, detector excitation at 310 nm and emission at 370 nm were used[3].
Pharmacokinetic calculations and statistics The Cmax and tmax values were read directly from the concentration–time profile. The area under the concentration-time curve (AUC0→t) was calculated by the trapezoidal rule. The variance for the AUC0→twas estimated using the method of Yuan[4]. The absolute oral or nasal bioavailability of Hup A was calculated as the ratio of the AUCin (AUCoral) to the AUCiv:
Fin=(AUCin×Doseiv)/(AUCiv×Dosein)×100%
The ratio of the AUCbrain to the AUCplasma (drug targeting efficiency) was calculated to evaluate the brain targeting of the drug via 3 administration routes. The statistical differences were assessed using the unpaired Student's t-test.
Foral=(AUCoral×Doseiv)/(AUCiv×Dose oral)×100%
Results
Determination of Hup A in rat plasma and brain tissue by HPLC
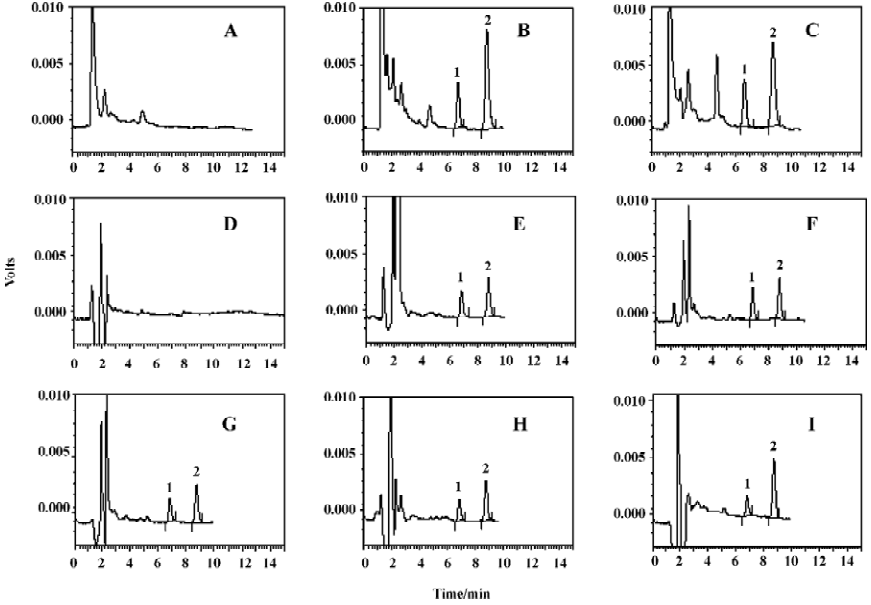
Separation and specificity Blank samples were chromatographically screened and there was no chromatographic interference with Hup A or IS; the retention times of Hup A and IS were approximately 6.8 and 8.8 min, respectively. The chromatograph is shown in Figure 3.
Calibration and linearity The calibration curves of Hup A were prepared with drug-free plasma and brain tissue samples spiked with known amounts of the drug, utilizing the peak area ratio of Hup A to IS. The linear range of Hup A was 2.86‒285.71 ng/mL for the plasma, and 1.25‒125 ng/g for the brain tissue.
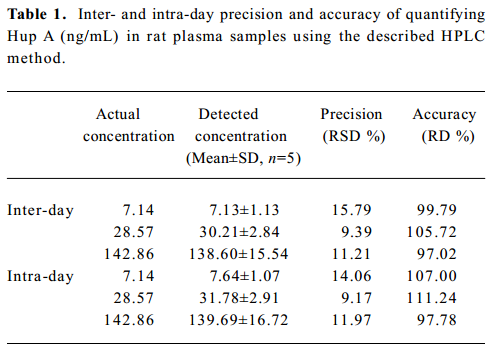
Precision and accuracy The inter- and intra-day precisions [relative standard deviation (SD)] and accuracy [relative deviation (RD)] are summarized in Tables 1 and 2.
Full table
Full table
Recovery For the plasma samples, the average extraction recoveries of Hup A for the low, medium and high QC were 84.59%, 79.10%, and 73.52%, respectively. For brain tissues samples, they were 71.47%, 68.21%, and 68.18%, respectively. The average extraction recoveries of IS were 86.64% and 65.38% in the plasma and brain samples.
Pharmacokinetic analysis and brain tissue distribution of Hup A
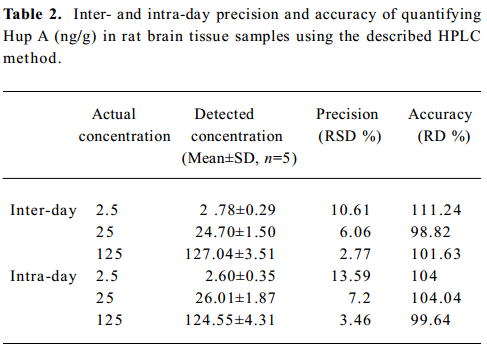
Rats plasma and brain tissue distribution of Hup A The mean brain tissue and plasma concentration-time profiles of Hup A in male rats following a single dose of the nasal in situ gel, the iv injection and the oral formulation are illustrated in Figure 4.
Following in administration of the nasal in situ gel at the dose of 166.7 µg /kg, the AUC0→6 h of Hup A in the cerebrum, hippocampus, cerebellum, left olfactory bulb, right olfactory bulb and plasma were 113.45±10.59 ng·h·g-1, 101.69±9.20 ng·h·g-1, 101.52±9.47 ng·h·g-1, 376.58±36.81 ng·h·g-1, 322.51±31.49 ng·h·g-1, and 202.30±18.86 µg·h·L-1, respectively.
The AUC0→6 h of Hup A in the cerebrum, hippocampus, cerebellum, olfactory bulb and plasma following iv dose of 166.7 µg /kg were 76.83±7.76 ng·h·g-1, 76.55±7.02 ng·h·g-1, 99.88±9.64 ng·h·g-1, 326.39±40.53 ng·h·g-1, and 209.71±18.52 µg·h·L-1, respectively; and those after oral formulation at the dose of 500 µg /kg were 124.21±10.95 ng·h·g-1, 141.38±18.74 ng·h·g-1, 161.56±21.34 ng·h·g-1, 364.06±25.52 ng·h·g-1, and 256.48±29.42 µg·h·L-1, respectively.
The absolute nasal bioavailability in the cerebrum, hippocampus, cerebellum, left olfactory bulb, right olfactory bulb and plasma were 147.7%, 132.8%, 101.6%, 115.4%, 98.8%, 96.5%, respectively. The absolute oral bioavailability in the cerebrum, hippocampus, cerebellum, olfactory bulb and plasma were 53.9%, 61.6%, 53.9%, 37.2%, and 40.8% respectively.
The AUC0→6 h of HupA in the plasma and all brain tissue samples after intranasal administration of the Hup A in situ gel were significantly higher (P<0.01) than those administered with the oral formulation.
The uptake extent of Hup A into the cerebrum and hippocampus 6 h after intranasal administration of the Hup A in situ gel to the rats was significantly higher (P<0.01) than that after iv administration of the injection. And there was no difference in the AUC0→6 h in the cerebellum, olfactory bulb and blood samples from rats receiving the drug in the form of iv injection and nasal in situ gel.
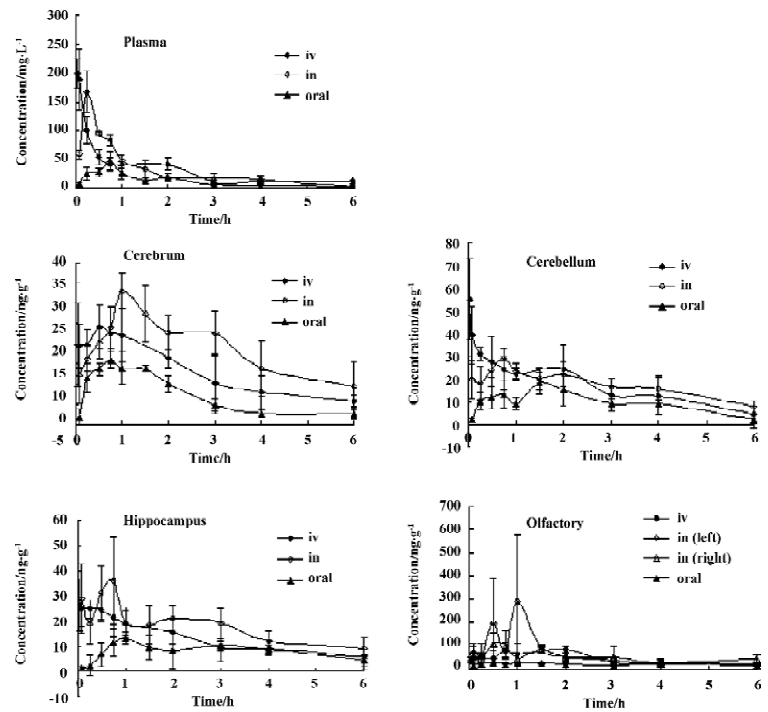
AUCbrain/AUCplasma of Hup A after iv administration of the injection and intranasal administration of the in situ gel to male rats The AUC brain/AUCplasma of Hup A, as the drug targeting efficiency (DTE), after a single dose of the iv injection and the nasal in situ gel are illustrated in Figure 5. Compared with intravenous injection, the intranasal administration of the in situ gel produced significantly higher (P<0.05) levels of the AUCbrain/AUCplasma in the left olfactory bulb over 0.083?6 h, and in the right olfactory bulb over 0.083-1 h. The AUCbrain/AUCplasma of Hup A in the cerebrum and hippocampus following intranasal dose is significantly higher (P <0.05) than the iv dose, at 0.083, 3, 4, and 6 h.
Discussion
In situ gel is liquid-like in vitro which can be administered easily as a drop or by a spray device and become semi-solid as soon as there is contact with the mucosa. Low viscosity of the formulations is required for targeting the drug to the olfactory mucosa, and the gel can prolong the time of the drug in the nasal cavity so that it enhances the drug absorbance. Thus, the nasal in situ gel should have a prospective application.
Intranasal administration of the Hup A in situ gel significantly increased the distribution of the drug into the rat brain tissue, especially into the cerebrum and hippocampus. The absolute oral bioavailability of Hup A in the plasma and different brain regions were all lower than the absolute nasal bioavailability (P<0.01). The nasal delivery of the Hup A in situ gel, therefore, showed a viable alternative to the oral formulation.
Figure 4 shows that the decrease of Hup A in the brain tissue was slower than that in the plasma, which was the same as the results reported by Liu X et al[5].
It is believed that drugs uptake into the brain from the nasal cavity via 2 different pathways. One is that drugs administered by the nasal route may enter the systemic circulation and subsequently reach the brain by crossing the BBB. The other is that drugs may permeate the brain directly via the olfactory region[6]. We can deduce that the amount of drugs in the brain tissue after nasal application attributes to these 2 parts. The AUC0→6 h after the intranasal administration of the Hup A in situ gel was 1.5 times higher than that after iv administration of the injection in the cerebrum and1.3 times higher than that in hippocampus. There were no differences in the AUC0→6 h in the cerebellum, olfactory bulb and blood samples from rats receiving the drug by either route. The absolute nasal bioavailability of Hup A in the plasma was 96.5%. For the lipophilic and small molecule weight character of the drug, intranasal application of the Hup A in situ gel resulted in similar AUCplasma to the iv injection and considerable quantity of Hup A delivered to the brain along the nose-blood-brain pathways. Animals receiving iv Hup A therefore provided a measure of Hup A penetration into the central tissues expected from the bloodstream after the intranasal administration of the in situ gel. The difference in brain tissues was ascribed to targeted delivery with intranasal administration of the Hup A in situ gel. The excessive part of the drug in the brain tissues following intranasal, rather than iv doses, represented the brain AUC fraction contributed by the direct nose to brain pathway.
The direct nose to brain pathway could also be demonstrated by comparing the AUCbrain/AUCplasma of Hup A after iv administration of the injection and that after intranasal administration of the in situ gel to male rats. The AUCbrain/AUCplasma of Hup A in the left olfactory bulb following the intranasal dose was significantly higher (P<0.05) than the iv dose at 0.083?0.75 h, implying the potential for Hup A into the central tissue through the nose-brain pathway. The AUCbrain/AUCplasma of Hup A in the cerebrum and hippocampus following intranasal dose was significantly higher (P<0.05) than the iv dose, at 0.083, 3, 4, and 6 h. We could deduce that Hup A could be absorbed in the brain tissue through the olfactory bulb in the nose to brain pathway, but it could not be explained that the AUCbrain/AUCplasma of Hup A in the cerebellum following iv dose was significantly higher (P<0.05)than intranasal dose, at 0.5, 0.75, and 1 h.
Additionally, it was necessary to anesthetize the rats in order to ensure the exact administration dose. However, the rat intranasal administration method still needs improvement, and further research on the effects of the anesthetized state on brain distribution of Hup A in in administration is needed.
The present study implicated a direct pathway for a fraction of the drug into brain following intranasal administration of the Hup A in situ gel. The intranasal delivery showed a viable, non-invasive strategy for delivering the drug into the brain.
References
- Zangara A. The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease. Pharmacol Biochem Behav 2003;75:675-86.
- Bagger MA, Bechagaard E. The potential of nasal application for delivery to the central brain ? a microdialysis study of fluorescein in rats. Eur J Pharm Sci 2004;21:235-42.
- Yue P, Zhao Y, Tao T. Determination of Huperzine A concentration in cerebrospinal fluid of rats by HPLC-fluorescence. Zhongguo yao xue za zhi 2005; 40: 1503-5.
- Yuan J. Estimation of variance for AUC in animal studies. J Pharm Sci 1993;82:761-3.
- Liu X, Li D, Wang XL, Chen CL. Determination and the pharmacokinetic study of huperzine A in mouse plasma and brain by HPLC/MS/MS. Fudan Univ J Med Sci 2006;33:247-50.
- Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 2000;11:1-18.