Urotensin II inhibits carotid sinus baroreflex in anesthetized male rats
Introduction
Urotensin II (UII) is a vasoactive ‘somatostatin-like’ cyclic peptide which was originally isolated from fish spinal cords, and has recently been cloned from man[1,2]. The orphan receptor GPR14, a G-protein-coupled receptor first cloned from rat, has been defined as the specific receptor for UII[3–5]. Positive immunoreactive staining for human UII and GPR14 mRNA was detected in both atrial and ventricular tissues[3–6]. These studies indicate that UII might act as one of the endogenous modulators of cardiac function. The cardiovascular action of UII has been examined in a number of in vitro and in vivo systems, sometimes with contrasting responses depending on the species and vessel studied. In mammals, UII was first reported to have a vasoconstrictor action in isolated rat thoracic aorta denuded of endothelium[7], an action that was later shown to be more potent than that of endothelin[3]. UII was also shown to cause vasoconstriction in endothelium-denuded vessels from various species including cynomolgus monkey thoracic aorta[3], dog coronary artery[3], and human radial artery[8]. In contrast, UII caused vasodilation in human small pulmonary arteries and abdominal adipose tissue arteries[9]. In addition, the cardiovascular responses to UII in vivo are equally variable. Systemic administration of human UII to monkeys elicited a fall in cardiac output, severe myocardial contractility depression, and fatal circulatory failure[3]. Both in anesthetized and conscious rats, UII administered iv decreased arterial pressure[10,11], and hypotension was associated with mesenteric and hindquarter vasodilatation[11], while intracerebroventricular injection UII caused arterial pressure and heart rate increase in conscious rats[12]. Even less clear is the role of UII in states of cardiovascular disease, but to date, increased plasma UII has been described in 3 states of volume overload, such as kidney failure[6], heart failure[13], and diabetes[14]. Thus, it is important to have more detailed understanding of the actions and the role of UII in cardiovascular control.
It is well known that baroreflex is a major mechanism in modulating blood pressure (BP). Whether UII affects the carotid sinus baroreflex (CSB) remains to be clarified. The aims of our study were to observe the action of UII on the isolated CSB and to elucidate the mechanism involved.
Materials and methods
Drugs UII, verapamil, BIM-23127, and L-NAME were purchased from Sigma (St Louis, MO, USA). All drugs were dissolved in distilled water and prepared freshly.
General surgical procedure Male Sprague-Dawley rats (320±20 g), obtained from the Experimental Animal Center of Hebei Province (Hebei, Shijiazhuang, China), were anesthetized with 25% urethane (1.0 g/kg, ip). The trachea was cannulated for ventilation. The right femoral artery was cannulated for recording BP with a transducer (MPU-0.5A, Nihon Kohden, Tokyo, Japan). Body temperature was maintained at 37–38 ºC throughout the experiment.
Perfusion of left isolated carotid sinus The perfusion of the isolated carotid sinus area was carried out with a method modified by our laboratory[15,16]. Carotid sinus areas were fully exposed by turning rostrally the trachea and esophagus. Sternohyoideus muscles and superior laryngeal nerves were cut, then the bilateral aortic nerves, right carotid sinus nerve, cervical sympathetic nerves, and recurrent laryngeal nerves were all sectioned. The common, external and internal carotid arteries and smaller arteries originating from these vessels were exposed and ligated, while carefully leaving the left carotid sinus nerve undisturbed. Ligation of the occipital artery at its origin from the external carotid artery excluded chemoreceptors from the isolated carotid sinus, thereby preventing chemoreceptor activation secondary to the decrease of carotid sinus pressure. Plastic catheters were inserted anterograde into the left common carotid artery (inlet tube) and retrograde into the external carotid artery (outlet tube). The carotid sinus was then perfused with warm (37 ºC) oxygenated modified Krebs-Henseleit (K-H) solution (in mmol/L; NaCl 118.0, KCl 4.7, CaCl2 2.5, MgSO4 1.6, KH2PO4 1.2, NaHCO3 25, glucose 5.6, pH 7.35–7.45), bubbled with 95% O2 and 5% CO2. The intrasinus pressure (ISP) was monitored by using a pressure transducer (MPU-0.5A, Nihon Kohden, Tokyo, Japan) connected to the inlet tube. The ISP was controlled by using a peristaltic pump.
After perfusion of the left carotid sinus, the ISP was kept at 100 mmHg for 20 min and then was lowered to 0 mmHg rapidly. From this point, the ISP was elevated to 250 mmHg in the form of a pulsatile ramp by regulating the speed of the peristaltic pump, which was automatically controlled by a program designed in our laboratory[15]. It took 0.5 min for the ISP to be increased from 0 to 250 mmHg. The ISP and BP were simultaneously recorded on a polygraph (RM-6240, Chengdu Instrument Factory, Sichuan, China). This process was repeated at an interval of 5 min to check the stability of the baroreflex. The reproducibility of the experimental setup was confirmed by the recurrent drop of BP in response to the increase in ISP.
Experimental protocols By perfusing the left carotid sinus with K-H solution and elevating the ISP, a functional curve for the ISP–BP relation was constructed, and the functional parameters of baroreflex, such as threshold pressure (TP), saturation pressure (SP), equilibrium pressure (EP), peak slope (PS), reflex decrease of BP (RD), and operating range (OR) were determined. TP was the ISP at which BP began to decrease in response to the increase of ISP. SP was the ISP at which BP just showed no further reflex decreases with an increase in ISP. OR was calculated as the SP minus TP.
Before administration of drugs, the K-H solution was used as a control. Four experimental treatments were used: (1) to test the effect of UII on carotid baroreflex (n=24), the ISP was fixed at 100 mmHg for 20 min with K-H solution as a control, and the baroreflex parameters were measured. Then K-H solution containing UII (3, 30, 300, or 3000 nmol/L) was used to perfuse the isolated carotid sinus for 50 min; afterward the parameters were measured again. Finally, the carotid sinus was perfused with K-H solution to wash out the UII; (2) to test the effect of verapamil (10 µmol/L) on the actions of UII (n=6), baroreflex parameters were examined following application of UII (300 nmol/L) before and after pretreatment with verapamil for 20 min; (3) to test the effect of BIM-23127 (3.0 µmol/L) on the actions of UII (n=6), baroreflex parameters were examined following the application of UII (300 nmol/L) before and after pretreatment with BIM-23127 for 20 min; and (4) to test the effect of NG-nitro-L-arginine methyl ester (L-NAME, 100 µmol/L) on the actions of UII (n=6), L-NAME were added into a K-H solution and used to perfuse the isolated carotid sinus after the baroreflex parameters of the control were recorded; 20 min later the baroreflex parameters were recorded again. Finally, the carotid sinus was perfused with K-H solution containing L-NAME and UII (300 nmol/L) to check the carotid baroreflex activity.
Data analysis All data were expressed as mean±SD. The differences between groups of means were assessed by one-way ANOVA and further analyzed using the Student-Newman-Kuels test. P<0.05 was considered statistically significant.
Results
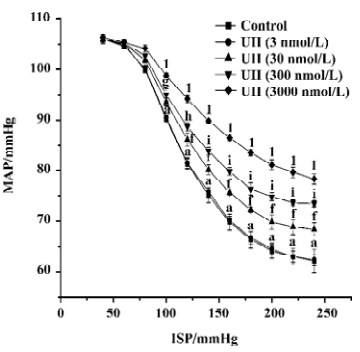
Effects of UII on carotid sinus baroreflex By perfusing the left carotid sinus with K-H solution and elevating ISP from 0 to 250 mmHg, the BP was reflexly decreased. UII altered the baroreflex parameters, which appeared approximately 30 min after UII started, and disappeared after 30−60 min perfusion of washout. Compared with the control group, UII at the concentration of 3 nmol/L had no effect on the CSB, while at concentrations of 30, 300, and 3000 nmol/L, it inhibited the CSB, shifting the functional curve of the baroreflex upward and to the right. There was a marked decrease in PS and RD in BP. The effects were concentration dependent (Table 1, Figure 1). The functional curves were shown in Figure 1. These results indicated that UII exerted an inhibitory action on the carotid baroreflex (Figure 2).
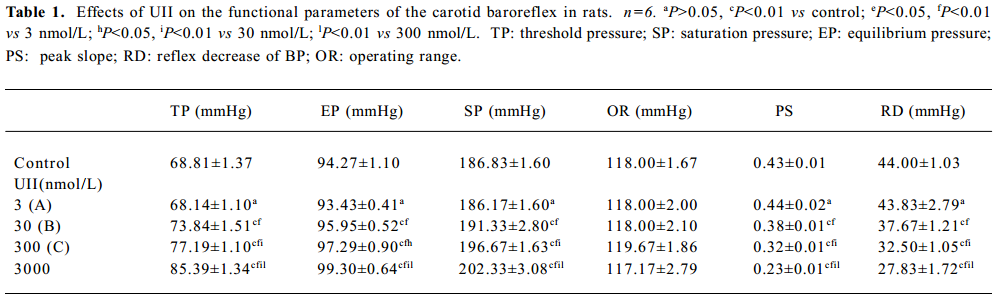
Full table
Effect of verapamil on the actions of UII Verapamil (10 µmol/L) did not affect the functional parameters of the carotid baroreceptor, but it partially blocked the actions of UII (300 nmol/L, Table 2).
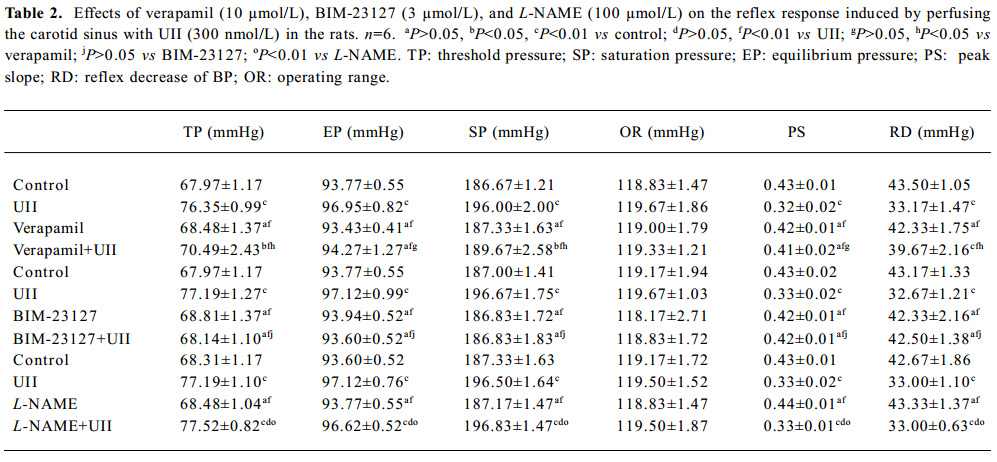
Full table
Effect of BIM-23127 on the actions of UII BIM-23127 (3.0 µmol/L), an antagonist of human and rat UII receptors, did not affect functional parameters of baroreflex alone, but it abolished the actions of UII (300 nmol/L) on the CSB.
Effect of L-NAME on the actions of UII L-NAME (100 µmol/L) did not affect the functional parameters of the carotid baroreceptor, and did not influence the effects of UII (300 nmol/L) either (Table 2).
Discussion
The present study showed for the first time that UII could inhibit the CSB in a dose-dependent manner. By perfusing the left isolated carotid sinus with UII, the functional curve of the CSB was shifted upward and to the right, with decreases in PS and RD and increases in TP, indicating the inhibitory action of UII on the CSB. It is well established that the arterial baroreceptors play an important role in the short-term control of cardiovascular activity. The inhibition of the CSB might be expected to weaken the ability to antagonize hypertension and destabilize BP.
It was demonstrated that baroreceptors would be excited when their ending was deformated by tissue tension[17], that is, wall tension σ, which by the Laplace’s relation is: σ=P⋅r/h (where P is the distending pressure, r is the radius of the vessel lumen, and h is the wall thickness). UII might directly induce arterial contraction and thus decrease r, so σ became smaller, and then the sensitivity of the baroreceptor was decreased. Thus, UII-induced inhibition of the CSB might have resulted from the contraction of the vessel. Indeed, it is known that UII is a vasoconstrictor, being even more potent than endothelin[3]. UII was also shown to stimulate the constriction of endothelium-denuded rat aorta and increase cytosolic Ca2+ levels within 10–20 s, which was partially blocked by the L-type calcium channel antagonist, verapamil[18]. In order to elucidate the relationship between the L-type calcium and the action of UII, we used verapamil. We found in the present study that pretreatment with verapamil partially blocked the effects of UII on the CSB, suggesting that the opening of the L-type calcium channel was involved in the action of UII on the CSB.
BIM-23127 was reported as a potent and competitive antagonist of both human UII and rat UII receptors, and was also considered as the most potent agent for reversing human UII-induced contractile tone in the rat isolated aorta[19]. In the present study, we found that BIM-23127 abolished UII action on the CSB. This result indicates that UII inhibits the CSB through the UII receptor.
UII was also reported as a vasodilator via release of one or more vasodilator factors such as nitric oxide (NO) in rat isolated aorta[20]. Since the endothelium is intact in the present study, UII may induce NO release from the carotid sinus. Increasing evidence have shown that NO may suppress the generation of action potential of baroreceptors[21,22]. NO suppressed Na+ current in baroreceptor neurons and activated the calcium-dependent K+ channels localized in vascular smooth muscles, then hyperpolarized baroreceptor neurons[21]. In order to test the role of NO plays, we tested the effect of L-NAME, a nonselective inhibitor of the NO synthase. Pretreatment with L-NAME did not affect the action of UII, thus indicating that locally-released NO was not involved in the effect of UII on the CSB. Thus, we propose that vasodilatation in response to UII is likely to have resulted from UII receptors on the endothelium, while constriction responses are mediated by UII receptors on smooth muscle cells.
In summary, the present study indicates that UII exerts an inhibitory action on the isolated CSB. Such an action of UII is predominantly mediated by the UII receptors in vascular smooth muscles, resulting in the opening of L-type calcium channels.
References
- Chartel N, Conlon JM, Collin F, Braun B, Waugh D, Vallarino M, et al. Urotensin II in the central system of the frog Rana ridibunda: immunohistochemical localization and biochemical characteriza-tion. J Comp Neurol 1996;364:324-39.
- Conlon J M, Yano K, Waugh D, Hazon N. Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J Exp Zool 1996;275:226-38.
- Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature 1999;401:282-6.
- Liu QY, Pong SS, Zeng ZZ. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem Biophys Res Commun 1999;266:174-8.
- Mori M, Sugo T, Abe M, Shimomura Y, Kurihara M, Kitada C, et al. Urotensin II is the endogenous ligand of a G-protein-coupled receptor, SENR (GPR14). Biochem Biophys Res Commun 1999;265:123-9.
- Totsune K, Taskahashi K, Arihara Z, Sone M, Stoh F, Ito S, et al. Role of urotension II in patients on dialysis. Lancet 2001;358:5-9.
- Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, et al. Differential vasoconstrictor activity of human urotension-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol 2000;131:1262-74.
- Maguire JJ, Kuc RE, Davenport AP. Orphan-receptor ligand human urotensin-II: receptor localization in human tissue and comparison of vasoconstrictor responses with endothelin-1. Br J Pharmacol 2000;131:441-6.
- Stirrat A, Gallagher M, Douglas SA, Ohlstein EH, Berry C, Kirk A, et al. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am J Physiol Heart Circ Physiol 2001;280:925-8.
- Gibson A, Wallace P, Bern HA. Cardiovascular effects of urotensin II in anesthetized and pitched rats. Gen Comp Endocrinol 1986;64:435-9.
- Gardiner SM, March JE, Kemp PA, Davenport AP, Bennet T. Depressor and regionally selective vasodilator effects of human and rat urotensin II in conscious rats. Br J Pharmacol 2001;132:1625-9.
- Lin Y, Tsuchihashi T, Matsumura K, Abe I, Iida M. Central cardiovascular action of urotensin II in conscious rats. J Hypertens 2003;21:159-65.
- Richards AM, Nicholls MG, Lainchbury JG, Fisher S, Yandle TG. Plasma urotensin II in heart failure. Lancet 2002;360:545-6.
- Totsune K, Takahashi K, Arihara Z, Sone M, Ito S, Muramaki O. Increased plasma urotensin II levels in patients with diabetes mellitus. Clin Sci 2003;104:1-5.
- Yi XL, Fan ZZ, He RR. An automatic system controlled by computer for carotid sinus perfusion. Chin J Appl Physiol 1993;9:156-9.
- Wu YM, He RR. Bradykinin inhibits carotid sinus baroreflex in anesthetized rats. Acta Physiol Sin 1999;51:303-9.
- Arndt JO. Baroreceptors: morphology and mechanics of receptor zones and sischarge properties of baroafferents. In: Zucker IH, Gilmore JP. editors. Reflex control of the circulation. Boston: CRC Press; 1991. p 103–8.
- Tasaki K, Hori M, Ozaki H, Karaki H, Wakabayashi I. Mechanism of human urotension II-induced contraction in rat aorta. J Pharmacol Sci 2004;94:376-83.
- Herold CL, Behm DJ, Buckley PT, Foley JJ, Wixted MM, Douglas SA. The neuromedin B receptor antagonist, BIM-23127, is a potent antagonist at human and rat urotensin-II receptors. Br J Pharmacol 2003;139:203-7.
- Gibson A. Complex effects of Gillichthys urotensin II on rat aortic strips. Br J Pharmacol 1987;91:205-12.
- Li Z, Chapleau MW, Bates JN, Bielefeldt K, Lee HC, Abboud FM. Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. Neuron 1998;20:1039-49.
- Chapleau MW, Hajduczok G, Sharma RV, Wachtel RE, Cunningham JT, Sullivan MJ, et al. Mechanisms of baroreceptor activation. Clin Exp Hypertens 1995;17:1-13.
