The susceptibility of ventricular arrhythmia to aconitine in conscious Lyon hypertensive rats1
Introduction
Clinical reports have shown that arterial hypertension is correlated with a high risk of sudden cardiac death due to fatal ventricular arrhythmias[1,2]. However, few experimental studies were performed regarding the relationship between hypertension and the risk of ventricular arrhythmias, thus little information is available regarding the underlying mechanisms.
Arterial baroreflex (ABR) is very important in the regulation of cardiovascular activities. It was found that ABR function, expressed as baroreflex sensitivity (BRS), was impaired in hypertension[3,4]. In our previous studies, it was found that impaired ABR function, induced by anesthesia and sinoaortic denervation, led to an increased susceptibility of ventricular arrhythmia to aconitine, and an intact ABR function is necessary for preventing drug-induced ventricular arrhythmias[5]. In addition, ABR function is an important determinant of the magnitude of blood pressure (BP) fluctuation, and a sound ABR function helps to maintain a stable BP[6]. Therefore, it is logical to expect that the dysfunction of ABR and the abnormal hemodynamics may be associated with the susceptibility of ventricular arrhythmia to an arrhythmogenic agent in hypertensive rats. Aconitine is a highly cardiotoxic agent which is known to suppress the inactivation of voltage-dependent Na+ channels by binding to neurotoxin binding site 2 of the α-subunit of the channel protein. It is widely used in animals to produce experimental arrhythmia, and plays an important role in evaluating the antiarrhythmic effects of new drugs[7,8]. Accordingly, the present work was designed to investigate the relationship between BP, blood pressure variability (BPV), BRS, and the susceptibility of ventricular arrhythmia to aconitine in Lyon hypertensive rats (LH). Considering the fact that the baroreflex function was largely inhibited by anesthesia[9,10], the present experiments were carried out in conscious animals.
Materials and methods
Animals Male 20-week-old LH and Lyon low blood pressure rats (LL) were provided by the Animal Center at our institute. All the rats were housed within controlled temperature (23–25 °C) and lighting (8:00–20:00 light, 20:00–8:00 dark), and with food and tap water available ad libitum. All the animals used in this work received humane care in compliance with institutional animal care guidelines.
BP measurement Systolic BP (SBP), diastolic BP (DBP) and the heart period (HP) of the rats were continuously recorded using the previously described technique[11,12]. Briefly, the rats were anesthetized with a combination of ketamine (40 mg/kg) and diazepam (6 mg/kg). A floating polyethylene catheter was inserted into the lower abdominal aorta via the left femoral artery for BP measurement, while another catheter was placed into the left femoral vein for intravenous injection. The catheters were exteriorized through the interscapular skin. After a 3 d recovery period, the animals were placed in individual cylindrical cages containing food and water for BP recording. The aortic catheter was connected to a BP transducer via a rotating swivel that allowed the animals to move freely in the cage. After about 4 h habituation, the BP signal was digitized by a microcomputer. SBP, DBP and HP values from every heartbeat were determined online. The mean values and standard deviation of these parameters during a period of 4 h were calculated. The standard deviation was defined as the quantitative parameter of BPV, that is, systolic BPV (SBPV), diastolic BPV (DBPV), and HP variability (HPV).
BRS measurement BRS was measured using the previously described method[3,13]. Briefly, the principle of this method is to measure the prolongation of HP in response to an elevation of BP. A bolus injection of phenylephrine was used to induce an elevation of SBP between 20–40 mmHg. HP was plotted against SBP for linear regression analysis and the slope of SBP-HP was expressed as BRS (ms/mmHg).
Arrhythmia induced by aconitine in anesthetized LH As described previously[5], under anesthesia with a combination of ketamine (50 mg/kg) and diazepam (5 mg/kg), the cathe-ters were inserted into the femoral artery and femoral vein using aforementioned means. Four electrodes were fixed subcutaneously at 4 extremities for electrocardiogram (ECG) recording. The aortic catheter and electrocardiogram electrodes were connected to the computerized BP and ECG monitoring systems. Aconitine (provided by E. Merck, AG Darmstadt, Germany) was then infused via the femoral vein catheter with a micro-infusion pump at a velocity of 0.2 mL/min (10 mg/mL in saline). BP and ECG were simultaneously recorded during the period of aconitine infusion until death of the animal[5,14]. The time when aconitine was administered and arrhythmias occurred was recorded. The threshold of aconitine required for producing ventricular premature beat (VPB), ventricular fibrillation (VF) and cardiac arrest (CA) were determined according to the following formula: threshold (µg/kg) for VPB=10 µg/mL×0.2 mL/min×time required for inducing VPB (min)/bodyweight (kg)=2 µg/min×time (min)/bodyweight (kg). The representative tracings of SBP and ECG in the anesthetized LH rats are shown in Figure 1.
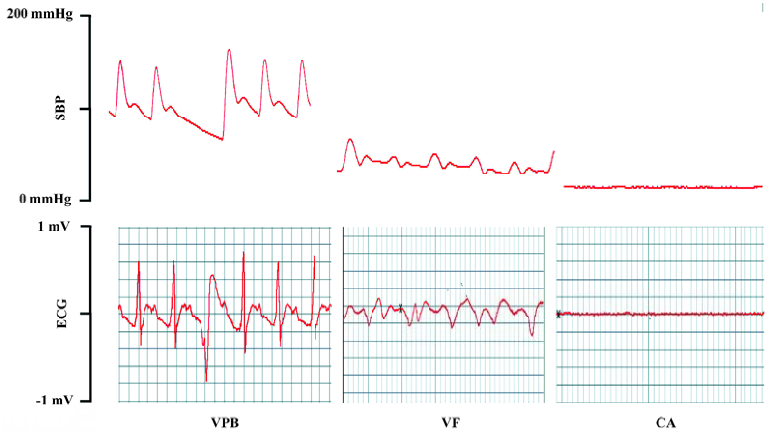
Arrhythmia induced by aconitine in conscious rats BP was recorded with the technique mentioned in the BP measurement section. When BP was stable, aconitine was infused via the fermoral vein catheter with a micro-infusion pump at a speed of 0.2 mL/min (10 µg/mL in saline) in conscious, unrestrained rats[5,14]. BP was monitored during the entire period of aconitine infusion. The threshold of aconitine required for producing cardiac VPB, VF, and CA was determined using the same formula as for the anesthetized rats. The characteristic of premature beat, ventricular fibrillation and cardiac arrest obtained in blood pressure tracings of the conscious rats was verified with that of electrocardiograms in anaesthetized rats.
Statistical analysis Data are expressed as mean±SD. Comparisons between groups were made by Student’s t-test. The relationships between the threshold of aconitine and hemodynamic parameters were analyzed by classic univariate correlation analysis. P<0.05 was considered statistically significant.
Results
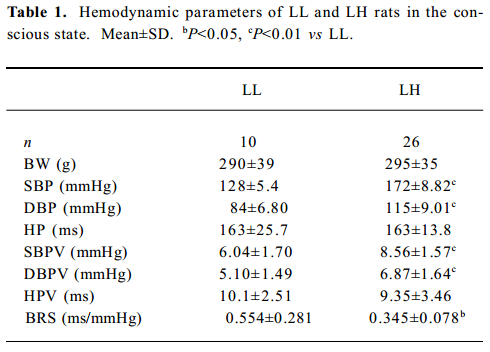
Hemodynamic parameters in conscious LL and LH rats Compared with the LL rats, the LH rats possessed signi-ficantly higher SBP (+34%, P<0.01), DBP (+37%, P<0.01), SBPV (+42%, P<0.01) and DBPV (+35%, P<0.01) and lower BRS (-38%, P<0.01). No obvious change was found in body weight, HP and HPV between the LL and LH rats (Table 1).
Full table
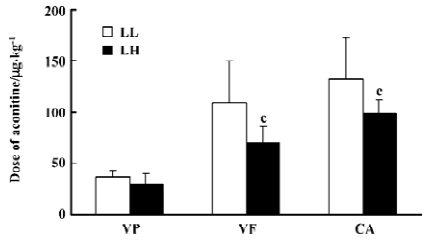
Threshold of aconitine required for arrhythmia in conscious LL and LH rats Compared with the LL rats, the LH rats displayed a significantly lower threshold of aconitine dose required for VF (LH: 70.5±16 vs LL: 109.0±41.2 mg/kg; P<0.01) and CA (LH: 92.0±13.9 vs LL: 132±40.4 mg/kg; P<0.01). The threshold of aconitine required for ventricular premature beat was not different between the LL and LH groups (29.7±10.8 vs 36.5±6.60 mg/kg; P>0.05) (Figure 1).
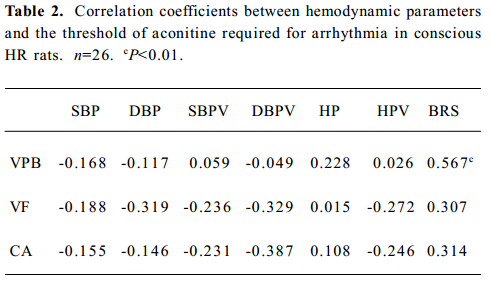
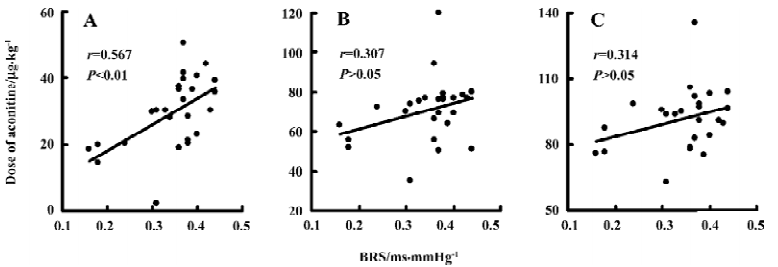
Relationships between hemodynamic parameters and the threshold of aconitine required for arrhythmia in conscious LH rats Relationships between hemodynamic parameters and the threshold of aconitine required for arrhythmia in conscious LH rats are shown in Table 2. It was found that all hemodynamic parameters studied were not correlated with the threshold of aconitine required for arrhythmia, with the exception of BRS, which was positively related to the threshold of aconitine required for VPB (r=0.567, P<0.01). Some examples of the correlations are shown in Figures 2,3.
Full table
Discussion
The present work demonstrated that the LH rats possessed significantly higher BP and BPV, lower BRS, and greater susceptibility of ventricular arrhythmias to aconitine than the LL rats.
It is well known that elevated BP contributes to a wide variety of complications associated with hypertension including stroke, heart failure and renal failure. During the early phase of such complications, end-organ damage, such as left ventricular hypertrophy and renal lesion are present. Indeed, BP level is a major determinant for hypertensive end-organ damage. In addition, clinical studies demonstrated that left ventricular hypertrophy in hypertensive subjects was associated with an increased prevalence of ventricular arrhythmias[15,16]. Therefore, we speculated that the elevated BP might contribute to the greater susceptibility of ventricular arrhythmias to aconitine in the LH rats. However, the present work showed that the BP level was not correlated with the threshold of aconitine required for arrhythmia in the LH rats. This result indicated that the BP level was not an independent determinant for ventricular arrhythmia onset in the LH rats.
It was found that the LH rats possessed significantly higher BPV when compared with the LL rats in the present work. Clinical observations suggest that BPV is related to organ damage in hypertensive patients[17–19]. In animal studies, organ damage induced by sinoaortic denervation were related to the high BPV, but not to the BP level[11,20]. As mentioned earlier, end-organ damage such as left ventricular hypertrophy in hypertensive subjects was associated with an increased prevalence of ventricular arrhythmias[15,16]. Accordingly, the role of BPV in hypertension-related abnormalities is imperative[21]. However, in the present work, BPV was not correlated with the threshold of aconitine required for arrhythmia in the LH rats. Therefore, BPV was not an independent determinant associated with ventricular arrhythmias in the LH rats.
Arterial baroreflex dysfunction is another characteristic of hypertension. It has been well recognized that BRS is impaired in hypertensive humans and animals[22–25]. Accordingly, we demonstrated that the LH rats had lower BRS than the LL rats. Previous studies suggest that BRS is an independent variable related to end-organ damage in hypertension[26]. Furthermore, we found that impaired arterial baroreflex function, induced by anesthesia and sinoaortic denervation, led to increased susceptibility of ventricular arrhythmia to aconitine, and intact baroreflex function is necessary for preventing drug-induced ventricular arrhythmias[5]. Nevertheless, the current study showed that BRS was only positively related to the threshold of aconitine required for ventricular premature beat, but not to those required for ventricular fibrillation and cardiac arrest. Hence, BRS was not one of the independent variables related to the susceptibility of ventricular arrhythmias to aconitine in the LH rats.
In conclusion, the LH rats possessed greater susceptibility to aconitine-induced ventricular arrhythmias when compared with LL rats. This greater susceptibility could not be attributed to any one of the hemodynamic parameters alone studied in LH rats. It is proposed that various hypertension-associated abnormalities, including the abnormal hemodynamics, may co-contribute to this vulnerability to ventricular arrhythmias.
Acknowledgement
The authors thank Prof Jean SASSARD at the University Claude Bernard (Lyon, France) for providing founder rats of Lyon hypertensive rats and Lyon low blood pressure rats.
References
- Novo S, Abrignani MG, Novo G, Nardi E, Dominguez LJ, Strano A, et al. Effects of drug therapy on cardiac arrhythmias and ischemia in hypertensives with LVH. Am J Hypertens 2001;14:637-43.
- Zehender M, Faber T, Koscheck U, Meinertz T, Just H. Ventricular tachyarrhythmias, myocardial ischemia, and sudden cardiac death in patients with hypertensive heart disease. Clin Cardiol 1995;18:377-83.
- Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: a quantitative method of assessing baroreflex sensitivity. Circ Res 1969;24:109-21.
- Parmer RJ, Cervenka JH, Stone RA, O’Connor DT. Autonomic function in hypertension: Are there racial difference? Circulation 1990;81:1305-11.
- Shu H, Yi-Ming W, Xu LP, Miao CY, Su DF. Increased susceptibility of ventricular arrhythmias to aconitine in anesthetized rats is attributed to the inhibition of baroreflex. Clin Exp Pharma-col Physiol 2004;31:249-53.
- Mancia G, Grassi G, Ferrari AU. Reflex control of the circulation in experimental and human hypertension. In: Zanchetti A, Mancia G, editors. Handbook of hypertension. Amsterdam: Elsevier Science BV; 1997. p 568–601.
- Ameri A. The effects of Aconitum alkaloids on the central nervous system. Prog Neurobiol 1998;56:211-35.
- Amran MS, Hashimoto K, Homma N. Effects of sodium-calcium exchange inhibitors, KB-R7943 and SEA0400, on aconitine-induced arrhythmias in guinea pigs in vivo, in vitro, and in computer simulation studies. J Pharmacol Exp Ther 2004;310:83-9.
- Shimokawa A, Kunitake T, Takasaki M, Kannan H. Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J Auton Nerv Syst. 1998;72:46-54.
- Kotrly KJ, Ebert TJ, Vucins EJ, Roerig DL, Stadnicka A, Kampine JP. Effects of fentanyl-diazepamnitrous oxide anaesthesia on arterial baroreflex control of heart rate in man. Br J Anaesth 1986;58:406-14.
- Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy by sinoaortic denervation. J Hypertens 2002;20:1865-72.
- Miao CY, Xie HH, Zhan LS, Su DF. Blood pressure variability is more important than blood pressure level in determination of end-organ damage in rats. J Hypertens 2006;24:1125-35.
- Xie HH, Miao CY, Jiang YY, Su DF. Synergism of atenolol and nitrendipine on hemodynamic amelioration and organ protection in hypertensive rats. J Hypertens 2005;23:193-201.
- Miao CY, Xu LP, Liu JG, Xie HH, Yuan WJ, Su DF. Frequent ventricular premature beats increase blood pressure variability in rats. Acta Pharmacol Sin 2004;25:545-53.
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998;32:1454-9.
- Novo S, Barbagallo M, Abrignani MG, Nardi E, Di Maria GU, Longo B, et al. Increased prevalence of cardiac arrhythmias and transient episodes of myocardial ischemia in hypertensives with left ventricular hypertrophy but without clinical history of coronary heart disease. Am J Hypertens 1997;10:843-51.
- Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension 2000;36:901-6.
- Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pres-sure variability. J Hypertens 2005;23:505-11.
- Sander D, Kukla C, Klingelhofer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3-year follow-up study. Circulation 2000;102:1536-41.
- Parati G, Lantelme P. Blood pressure variability, target organ damage and cardiovascular events. J Hypertens 2002;20:1725-9.
- Liu JG, Xu LP, Chu ZX, Miao CY, Su DF. Contribution of blood pressure variability to the effect of nitrendipine on end-organ damage in spontaneously hypertensive rats. J Hypertens 2003;21:1961-7.
- Myredal A, Gao S, Friberg P, Jensen G, Larsson L, Johansson M. Increased myocardial repolarization lability and reduced cardiac baroreflex sensitivity in individuals with high-normal blood pressure. J Hypertens 2005;23:1751-6.
- Milan A, Mulatero P, Williams TA, Carra R, Schiavone D, Martuzzi R, et al. Bradykinin B2 receptor gene (-58T/C) polymorphism influences baroreflex sensitivity in never-treated hypertensive patients. J Hypertens 2005;23:63-9.
- Ormezzano O, Poirier O, Mallion JM, Nicaud V, Amar J, Chamontin B, et al. A polymorphism in the endothelin-A receptor gene is linked to baroreflex sensitivity. J Hypertens 2005;23:2019-26.
- Su DF, Miao CY. Functional studies of arterial baroreflex in conscious rats. Acta Pharmacol Sin 2002;23:673-9.
- Shan ZZ, Dai SM, Su DF. Relationship between baroreceptor reflex function and end-organ damage in spontaneously hypertensive rats. Am J Physiol 1999;277:H1200-6.
