Role of PI3-K/Akt pathway and its effect on glial cell line-derived neurotrophic factor in midbrain dopamine cells1
Introduction
Glial cell line-derived neurotrophic factor (GDNF) was first isolated by virtue of its ability to induce dopamine (DA) uptake and cell survival in culture of embryonic ventral midbrain DA cells. Further experimental results have also revealed that GDNF may protect DA cells from injury by toxicity[1,2]. Although the biological effects of GDNF on DA cells have been studied extensively, the mechanisms underlying the role of GDNF are less known.
GDNF signals via multicomponent receptors that consist of the Ret receptor tyrosine kinase plus a glycosylphosphati-dylinositol-linked coreceptor named GDNF family receptor a1 (GFRa1). The binding of GDNF to Ret and GFRa1 induces Ret phosphorylation[3,4]. After phosphorylation, Ret induces the activation of several intracellular pathways, among which the phosphatidylinositol 3-kinase/Akt (PI3-K/Akt) pathway is of particular interest[5].
The PI3-K/Akt pathway is an important regulator of neuronal survival, both in central and peripheral nervous systems[6]. The PI3-K/Akt pathway is initiated by the activation of PI3-K, which in turn activates a cascade of downstream effectors including the serine/threonine kinase Akt[7]. The survival of sympathetic neurons of the superior cervical ganglion (SCG) induced by nerve growth factor (NGF) is critically dependent upon an intact PI3-K/Akt pathway[8]. GDNF is also able to activate the PI3-K/Akt pathway and promote the survival of SCG[9]. However, whether the PI3K/Akt pathway is involved in the survival/differentiation effects of GDNF on primary cultured DA cells is not yet well understood. Further studies show that both GFRa1 and Ret are present in midbrain DA cells[10].
In the present study, we examine the intracellular pathways activated by GDNF in DA cells in vitro, and whether the PI3-K/Akt pathway contributes to GDNF-induced DA cells survival/differentiation.
Materials and methods
Cell culture Primary DA cell culture was established from the ventral mesencephalic tissues of rat embryos as described previously[10]. Briefly, Sprague-Dawley pregnant rats were deeply anesthetized on gestational d 18, and fetuses were rapidly removed from the uterus and transferred to ice-cold Dulbecco’s modified Eagle’s medium (DMEM). The mesencephalic flexure enriched with DA cells was cut off from the fetal brain and minced into 1 mm×1 mm×1 mm pieces. After incubation for 15 min at 37 oC with 0.25% trypsin and 0.02% EDTA solution, the cells were separated by trituration through a syringe and passed through a 150 mesh sieve. The cell suspension was centrifuged for 5 min and then resuspended in complete medium [DMEM/F12 1:1, containing 10% fetal bovine serum (FBS), 4 mmol/L glutamine, 100 U/mL penicillin G sodium, and 100 µg/mL streptomycin sulfate]. The cells were plated at a density of 1.5×105 cells/well onto 24 well plates, which were pre-coated with 0.1 g/L poly-L-lysine for morphological or Western blot analysis, respectively. After 24 h in culture (1DIV), the media were replaced with serum-free medium (NeurobasalTM medium containing 2% B27 supplement, 4 mmol/L glutamine, 100 U/mL penicillin G sodium, 100 µg/mL streptomycin sulfate) and 10 ng/mL GDNF with or without wortmannin (Calbiochem, KY 12420, Germany). Wortmannin was used at 50 nmol/L, then on every other day, half of the same medium was replaced. On 6DIV, TH immunostaining was processed. Cultures were maintained at 37 oC in an atmosphere of 5% CO2/95% air and 100% relative humidity.
Tissue culture We used the embryonic d 18 Sprague-Dawley rats in this study. The pregnant Sprague-Dawley rats were deeply anesthetized, and the fetuses were rapidly removed from the uterus and transferred to ice-cold DMEM. The whole brain was rapidly removed and immediately chilled for 3–5 min in ice-cold DMEM, which had been preoxygen-ated in a 95% O2/5% CO2 incubator. The brains were then embedded in low-melting point agarose [2.5% in phosphate-buffered saline (PBS); type VII agarose, A9045; Sigma, St Louis, MO, USA], mounted onto the McIlwain tissue chopper stage. Coronal sections (400 µm) were cut, followed by separation in ice-cold DMEM, supplemented with 10% FBS and penicillin-streptomycin (100 U/mL, 100 µg/mL, respectively). Slices of interest were transferred onto 30 mm Millicell-CM (Millipore, Bedford, MA, USA) culture plate inserts (0.4 µm, 4 per well) in 6-well tissue culture plates containing 1.5 mL of the above growth medium. Slices containing a clearly defined midbrain(identified using the atlas of Paxinos and Watson 1986[11]) were used for the experiments. The control and treatment groups for a single trial were prepared at the same time and cultured for the same durations.
Throughout their growth period, the slices were kept at an interface between the growth medium and the humid atmosphere. The cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2/95% O2. After 24 h in the culture (1DIV), the culture media were replaced with a serum-free medium (NeurobasalTM medium containing 2% B27 supplement, 4 mmol/L glutamine, 100 U/mL penicillin G sodium, and 100 µg/mL streptomycin sulfate). Two hundred ng/mL GDNF with or without wortmannin was added. Two hundred nmol/L wortmannin was added 1 h prior to the GDNF addition. Then on every other day, half of the same medium was replaced. On 6DIV, immunohistochemistry and Western blotting were performed to detect tyrosine hydroxylase (TH).
To study the activation of the PI3-K/Akt pathways, the slices were cultured in serum-free medium. On 6DIV, Two hundred ng/mL GDNF with or without wortmannin was added. Akt phosphorylation was examined 30 min after GDNF was added.
Immunostaining On 6DIV, thin paraffin sections were deparaffinized and rehydrated, and the cells were fixed in 4% paraformaldehyde for 20 min. To block residual endogenous peroxidase activity, the sections and cells were incubated for 10 min with 3% hydrogen peroxide in PBS. After being washed 3 times with PBS for 5 min each, they were blocked with 1% bovine serum albumin (BSA) in PBS for 1 h at 37 °C, washed with PBS, and incubated with monoclonal mouse anti-rat tyrosine hydroxylase (TH) antibody at 1:3000 (Sigma, USA) overnight at 4 °C. After being washed 3 times with PBS, they were incubated with a biotinylated goat anti-mouse IgG (1:50; Sigma, USA) overnight at 4 °C. Peroxidase-conjugated streptavidin was added for 30 min at room temperature (RT) and the cultures were stained for peroxidase reaction by incubation with a mixture of diaminobenzidine and hydrogen peroxide for 5–10 min. The slides and cells were cleared and mounted with a microscope. Controls were prepared without the primary antibody.
To study the activation of the PI3-K/Akt pathway in our culture models, TH/p-Akt immunofluorescence double stain was processed. On 6DIV, the cells were treated as described earlier, and incubated with monoclonal mouse anti-rat TH antibody 1:3000 and monoclonal rabbit anti-mouse p-Akt antibody 1:1000 overnight at 4 °C. After being washed 3 times with PBS, they were incubated with Cy3-conjugated goat anti-mouse IgG or fluorescein(FITC) -conjugated goat anti-rabbit IgG for 2 h at 37 °C. After being washed 3 times with PBS, they were observed with a confocal microscope.
Western blotting After GDNF exposure on 6DIV, slice tissues were collected rapidly in ice-cold PBS, then homogenized at 4 °C in ice-cold lysis buffer [10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.9, 0.5 mmol/L MgCl2, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L O,O'-Bis(2-aminoethyl)ethyleneglycol-N,N,N',N'-tetraacetic acid, 50 mmol/L NaF, 5 mmol/L dithiothreitol, 10 mmol/L phosphoglycerol, 1 mmol/L Na3VO4, 1% NP40, 1 mmol/L benzamidine and enzyme inhibitors: 5 mg/mL phenylmethyl-sulfonyl fluoride, and 5 mg/mL each of pepstatin A, leupeptin, aprotinin]. After centrifugation, the supernatants were stored at -80 °C. Equal amounts (50 µg) of protein were separated by 10% SDS-PAGE, electrotransferred onto a nitrocellulose membrane and immunoblotted. Mouse anti-rat TH antibody (Sigma, USA) was used at 1:1000 dilution. Rabbit anti-mouse p-Akt antibody (Cell Signaling Technology, Beverly, MA, USA) was used at 1:2000 dilution. Goat anti-mouse AP (Sigma, USA) or goat anti-rabbit AP (Sigma, USA) as a secondary antibody were used at 1:5000 dilution. The negative control was prepared without the primary antibody, but including all other procedures. After blotting, the bands on the filter were scanned and analyzed with an image analyzer (LabWorks Software, UVP Upland, CA, USA). The optical density of the band in each lane was expressed as ‘fold’ versus that in the sham control lane in the same filter. To standardize the total protein content in each lane, membranes were incubated at RT with a mouse monoclonal antibody against pan-Akt (1:2000; Cell Signaling Technology, USA) for 1 h. Other procedures were the same as described earlier.
Data analysis The effects of GDNF on DA neuronal survival/differentiation, and the impact of the inhibition of PI3-K/Akt pathway on the actions of GDNF, were measured and quantified. First, the number of TH-ir cells per mm2 and the number of primary neurite of 60 randomly selected DA cells were used as the index of DA cell survival/differentiation. The DA cells were selected randomly in the right-up, left-up, right-down, left-down and central part of the different visual field, respectively. Second, the phosphorylation of p-Akt was used as an index of the activation of the PI3-K/Akt path-way. Results were compared by one-way ANOVA test using the SigmaStat32 statistical program.
Results
GDNF promoted the survival/differentiation of midbrain DA cells In the present study, both midbrain slice culture and cell culture, in which GDNF could promote the survival/differentiation of DA cells, were established as our experimental models. In each model, the identity of the DA cells in the culture was confirmed by the positive staining for TH.
The first step of our study was to decide the appropriate concentration of GDNF for the effective biological effect to promote the survival/differentiation of DA cells. In the cell culture model, the concentration of GDNF was 10 ng/mL, which is consistent with Horger’s report[12], while in the slice culture model the concentration was 200 ng/mL, which is consistent with our previous study.
Consistent with previous findings[1,13,14], the present results showed that GDNF promoted the survival and morphological differentiation of DA cells in the 2 culture models.
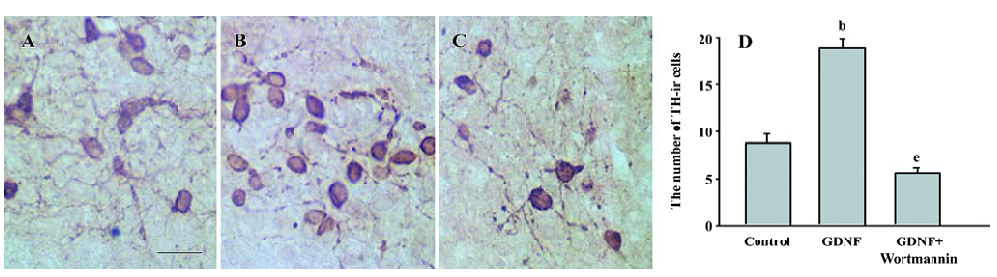
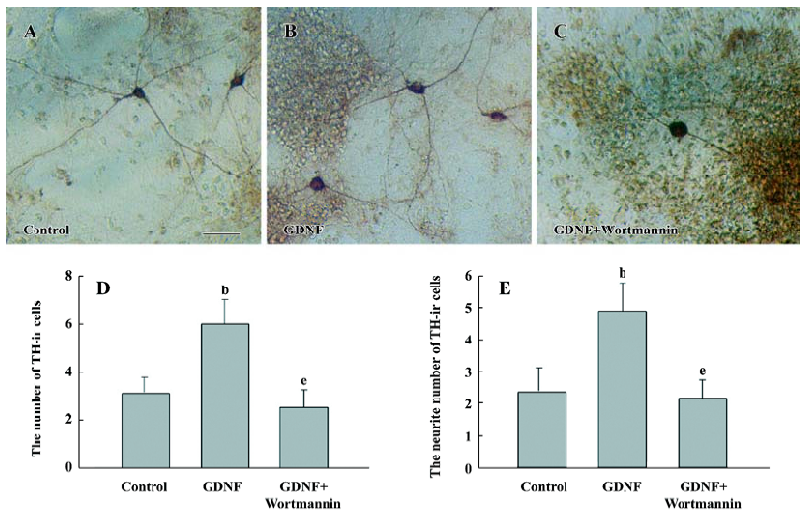
The results of immunostaining showed that the number of TH-ir cells per mm2 in the GDNF-treated group (18.63±0.95) was significantly more than that in the control of the slice culture (8.76±0.75; Figure 1), and that both the number of TH-ir cells per mm2 and the neurite number of TH-ir cells in the GDNF-treated group (6.01±0.43 and 4.89±0.46) were significantly more than that of the control of the cell culture (3.65±0.88 and 2.49±0.42; n=6; Figure 2).
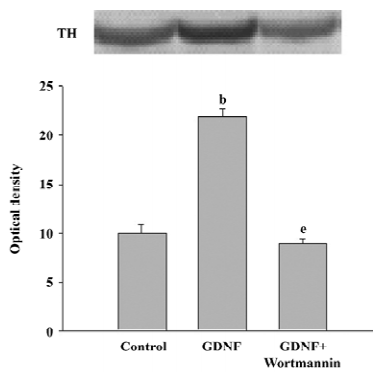
The results of Western blotting also showed that the level of TH expression in the GDNF-treated group was significantly higher than that of the control (n=3; Figure 3).
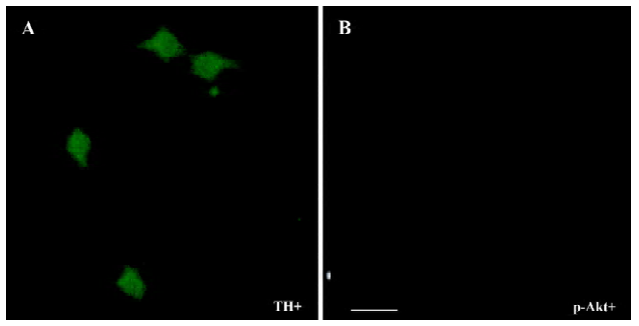
The PI3-K/Akt pathway was activated when GDNF exerted the survival/differentiation effect on DA cells We then explored the possible intracellular pathways underlying the effect of GDNF on DA cells. It had been demonstrated that the binding of GDNF to its receptors initiated several intracellular pathways, among which the PI3-K/Akt pathway was of particular interest. To test whether GDNF was able to activate the PI3-K/Akt pathway in our experimental models, the cells in the 2 cultures were stimulated with GDNF (10 ng/mL or 200 ng/mL) at 6DIV. Thirty minutes after the addition of GDNF into the media, the phosphorylation of Akt was examined by TH/p-Akt immunofluorescence double staining and by Western blotting. The results showed that p-Akt was expressed in the TH-ir cells (Figure 4) and the level of p-Akt expression in the GDNF-treated group was higher than that of the control (Figure 5). It was suggested that the PI3-K/Akt pathway was activated after GDNF treat-ment.
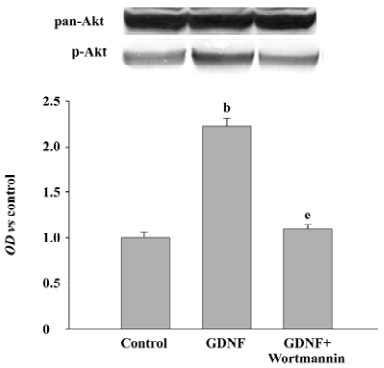
Survival/differentiation effect of GDNF on DA cells was abolished by the inhibitor of the PI3-K/Akt pathway Wortmannin was used to specifically block the PI3-K/Akt pathway. To further demonstrate the role of the PI3-K/Akt pathway in the survival/differentiation effect of GDNF on DA cells, GDNF (10 ng/mL or 200 ng/mL) with or without wortmannin was added to cultures at 1DIV. Wortmannin was plused into the medium 1 h before GDNF. Then on 6DIV, TH immunostaining and Western blotting were performed. The result of TH immunostaining showed that wortmannin not only blocked the phosphorylation of Akt induced by GDNF, but also abolished the effect of GDNF on neuronal survival/differentiation of DA cells. The number of TH-ir cells per mm2 in the PI3-K/Akt pathway-inhibited group (6.98±0.58) was significantly lower than that of the GDNF-treated group (18.63±0.95) in the slice culture (Figure 1). The number of TH–ir cells per mm2 and the neurite number of TH–ir cells in the PI3-K/Akt pathway-inhibited group (3.79±0.62 and 2.50±0.25) was significantly lower than that of the GDNF-treated group (6.01±0.43 and 4.89±0.46) in the cell culture (n=6; Figure 2). Western blot analysis of TH expression in such conditions confirmed the above results (n=3; Figure 3). The expression of TH decreased in the PI3-K/Akt pathway-inhibited group. These results suggest that the PI3-K/Akt pathway mediates the survival/differentiation effects of GDNF on DA cells.
Discussion
The study was conducted both on the cell culture and on the slice culture of the midbrain. The role of the PI3-K/Akt pathway in mediating the effect of GDNF on DA cells was explored. Our results showed that the PI3-K/Akt pathway was activated when GDNF promoted the survival/differentiation of DA cells; when the PI3-K/Akt pathway was blocked by wortmannin, the effect of GDNF on DA cells was abolished. The results suggest that the PI3-K/Akt pathway may be involved in mediating the survival/differentiation role of GDNF on DA cells.
Through binding to its receptors, GDNF may induce the activation of the extracellular regulated kinase (ERK)-mitogen-activated protein kinase (MAPK) and the PI3-K/Akt pathway[5]. The PI3-K/Akt pathway has been implicated in the survival-promoting mechanisms[15–18].
In the present work, we show that GDNF increases the phosphorylation of Akt, indicating that the PI3-K/Akt pathway is activated. To confirm the involvement of the PI3-K/Akt pathway in mediating the effect of GDNF, we treated cultured cells with wortmannin, which is the inhibitor of the PI3-K/Akt pathway. The results showed that when wortman-nin was added to the medium, the number of TH-ir cells and the neurite number of TH-ir cells dramatically decreased compared with the control, suggesting that the PI3-K/Akt pathway might play a striking role in mediating the survival/differentiation effect of GDNF on cultured DA cells. This is consistent with previous observations that the PI3-K/Akt pathway was found to mediate the survival effect of GDNF on cultured serum-starved spinal motor neurons, sympathetic neurons, and cerebellar granule cells[19]. Moreover, the role of the PI3-K/Akt pathway as a mediator of the trophic effect of several trophic factors has been described previously in the brain-derived neurotrophic factor-mediated survival of cultured cerebellar granule neurons[20] or spinal cord medial terminal nuclei[18], in NGF maintained PC12 or SGC cells[8,16], and in cerebellar granule neurons maintained with Insulin-like growth factor I[15,17]. However, the present work demonstrated the involvement of the PI3-K/Akt pathway in mediating the survival/differentiation process of GDNF on DA cells both in cell culture and in slice culture.
Opinions about the ERK-MAPK pathway have been perplexing. Some studies consider that both the PI3-K/Akt pathway and the ERK-MAPK pathway play a role in cell survival[21,22], while others think that the activation of the ERK–MAPK pathway is not involved in the cellular events directly related with cell survival. However, the activation of this pathway will be an important step in mediating neuronal differentiation[23]. It seems that different growth factors acting on different cell type may have different mechanisms to achieve certain actions; that further studies need to be conducted to uncover the real sense of these mechanisms.
Survival signals from various cell surface receptors activate PI3-K to phosphorylate the downstream effector Akt, which plays key roles in cellular processes such as glucose metabolism, cell proliferation, apoptosis, transcription, and cell migration. The exact role of activated Akt is determined by its downstream target. So the elucidation of the anti-apoptotic function of Akt signaling immediately precipitated an intensive search for downstream targets involved in cell survival. One possible downstream target on which this signaling cascade converges is transcription factor nuclear factor-κB (NF-κB). Upon the activation of Akt, NF-κB may activate the transcription of anti-apoptotic proteins such as the inhibitor of apoptosis proteins, c-IAP1 and c-IAP2.
In conclusion, our work demonstrated that the activation of the PI3-K pathway is involved in the effect of GDNF on cultured DA cells in both cell culture and slice culture models. The PI3-K/Akt pathway plays a pivotal role in DA neuronal survival/differentiation after GDNF stimulation. Gaining insight into the cellular mechanisms underlying the effects of GDNF may reveal cellular targets for treating Parkinson’s disease.
References
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993;260:1130-2.
- Shingo T, Date I, Yoshida H, Ohmoto T. Neuroprotective and restorative effects of intrastriatal grafting of encapsulated GDNF-producing cells in a rat model of Parkinson’s disease. J Neurosci Res 2002;69:946-54.
- Kjær S, Ibáñez CF. Identification of a surface for binding to the GDNF-GFR α1 complex in the first cadherin-like domain of RET. J Biol Chem 2003; 278: 47 898–904.
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci 2003;116:3855-62.
- Trupp M, Scott R, Whittemore SR, Ibáñez CF. RET-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem 1999; 274: 20 885–94.
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase. Mol Cell Biol 2001;21:893-901.
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, et al. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 2005;280:2737-44.
- Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci 1998;18:2933-43.
- Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM Jr. c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci 2001;21:1464-72.
- Neff F, Noelker C, Eggert K, Schlegel J. Signaling pathways mediate the neuroprotective effects of GDNF. Ann N Y Acad Sci 2002;973:70-4.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. NY: Academic Press; 1986.
- Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci 1998;18:4929-37.
- Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J Neurosci 2001;21:8108-18.
- Trupp M, Belluardo N, Funakoshi H, Ibáñez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-RET proto-oncogene, and gdnf receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci 1997;17:3554-67.
- Caro AA, Cederbaum AJ. Role of phosphatidylinositol 3-kinase/AKT as a survival pathway against CYP2E1-dependent toxicity. J Pharmacol Exp Ther 2006;318:360-72.
- Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 1995;267:2003-6.
- Miller TM, Tansey MG, Johnson EM, Creedon DJ. Inhibition of phosphatidylinositol 3-kinase activity blocks depolarization- and insulin-like growth factor I-mediated survival of cerebellar granule cells. J Biol Chem 1997;272:9847-53.
- Dolcet X, Egea J, Soler RM, Martin-Zanca D, Comella JX. Activation of PI 3-kinase, but not ERK MAP kinases, is necessary to mediate BDNF-induced motoneuron survival. J Neurochem 1999;73:521-31.
- Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneu-rons. J Neurosci 1999;19:9160-9.
- Li Z, Ding M, Thiele CJ, Luo J. Ethanol inhibits brain-derived neurotrophic factor-mediated intracellular signaling and activator protein-1 activation in cerebellar granule neurons. Neuroscience 2004;126:149-62.
- Xue LZ, Murray JH, Tolkovsky AM. The Ras/phosphatidyl-inositol 3-kinase and Ras/ERK pathways function as independent survival modules each of which inhibits a distinct apoptotic signaling pathway in sympathetic neurons. J Biol Chem 2000;275:8817-24.
- Li F, Omori N, Jin G, Wang SJ, Sato K, Nagano I, et al. Cooperative expression of survival p-ERK and p-Akt signals in rat brain neurons after transient MCAO. Brain Res 2003;962:21-6.
- Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, et al. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem 2005;33:29409-19.
