Biphasic effect of aspirin on apoptosis of bovine vascular endothelial cells and its molecular mechanism
Introduction
Aspirin (acetylsalicylic acid, ASA), a traditional analgesic-antipyretic drug, is now widely used in the prevention of cardiovascular diseases[1] and may even reduce the risk of Alzheimer’s disease[2] and cancers[3]. The classic mechanism of aspirin in the prevention of cardiovascular diseases has been known to inhibit the activity of cyclooxygenase (COX) in platelets, which leads to the reduction of prostaglandin and thromboxane A2 production[4]. However, recent studies show that the COX-inhibiting mechanism can not provide a full explanation of the cardiovascular protective effects of aspirin. Accumulating experimental evidence have indicated that many other effects of aspirin contribute to its cardiovascular protection, such as modulating the activity of the intracellular metabolism of ATP, inhibiting inducible nitric oxide synthase, modulating the activity of nuclear factor (NF)-kappa B and mitogen-activated protein kinases (MAPK)[5–7].
Apoptosis, or programmed cell death, is an active progress of cell elimination in physiological or pathological conditions[8]. Pathological apoptosis of endothelial cells not only damages the integrity of endothelium, but also affects the cytokine secretion of endothelial cells. Impaired endothelium function will then facilitate the formation of thrombus and promote atherogenesis[9]. However, inducing apoptosis of endothelial cells is potentially an effective strategy for blocking the progression of tumor development by inhibiting angiogenesis[10].
MAPK [extracellular signal-regulated kinase, Jun N-terminal kinase (JNK) and p38 MAPK] are well-known signal molecules involved in the regulation of cell proliferation and apoptosis. Among the 3 MAPK family members, p38 MAPK is activated by cell stress promoting cell apoptosis[11]. Aspirin has been shown to modulate MAPK expression in various cell types, including vascular endothelial cells[12–14]. In the present study, we investigated the direct effect of aspirin on the apoptosis of bovine aorta endothelial cells and the role of p38 MAPK in this process.
Materials and methods
Cell culture Bovine aortic endothelial cells (BAEC) were harvested as previously described[15] and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS), 100 kU/L benzyl- penicillin, and 100 mg/L streptomycin. Confluent cells were subcultured by trypsin digestion. Experiments were performed with cells from passages 4 to 10.
Cell viability assay Cell viability was measured through a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. BAEC were seeded into 24-well plates and grown to 80% confluence in DMEM with 10% FBS. After starved in phenol-free M1640 medium for 24 h, the cells were incubated with the respective test substance. MTT dissolved in phenol red free M1640 at a concentration of 5 g/L was added to the cell cultures at the end of the experi-ments. After incubation for 1 h at 37 oC, solubilization solution containing 10% (v/v) Triton X-100 and 0.1 mol/L HCl in isopropanol were added into the cell cultures to stop the reaction and dissolve formazan crystals. Metabolic activity was quantified by measuring light absorbance at 570 nm.
Morphologic determination and quantification of apopto-sis After the respective treatment, BAEC were stained with Hoechst 33258 and then observed under a fluorescence microscope. Under the fluorescence microscope, apoptotic cells with condensed or fragmented nuclei were easily distinguished from normal cells with intact nuclei. Quantification of apoptosis was routinely determined by counting the number of apoptotic cells: for each plate, we randomly chose 6 fields of view, and counted the total cells and apoptotic cells with a minimum number of 500 cells scored.
DNA electrophoresis At the end of experiments, the cells were harvested and DNA was extracted with standard phenol-chloroform extraction. Electrophoresis of DNA was performed in ethidium bromide-stained 1.5% agarose gel and visualized by exposure under UV light.
Western blotting BAEC cultured in 12-well culture plates were grown to 80%−90% confluence and then starved for 24 h in serum-free M1640 medium. After different treatment, the cells were scraped into lysis buffer containing (in mmol/L) NaCl 50, Na3VO4 2, phenylmethylsulfonyl fluoride 0.5, and HEPES 10 at pH 7.4, along with 0.01% Triton X-100 and 10 mg leupeptin. The cells were then disrupted by sonication, after which they were centrifuged for 20 min at 12 000×g to separate particular proteins from soluble fractions. The amount of proteins in each fraction was determined by the bicinchoninic acid (BCA) method.
Proteins (10 mg) were subjected to SDS-PAGE and electrophoretically transferred to a nitrocellulose membrane. The membranes were blocked with Tris-buffered saline Tween-20 (TBST) containing 5% bovine serum albumin (BSA) and immunoblotting was performed using either an antitotal-p38 MAPK antibody or antiphospho-p38 MAPK antibody. The membranes were then incubated with a second antibody conjugated to horseradish peroxidase and the blot was visualized by the Phototope Western Detection System (New England Biolabs, Ipswich, MA, USA) To control equal protein concentration in the experiments, 2 gels for each group were loaded parallely with the same protein samples and blotted for activated, phosphorylated p38 MAPK or total p38 MAPK. Bands of protein were quantitatively determined by thin-layer chromatography with Shimadzu Dual-Wavelength Chromato-Scanner (Model CS-930, Tokyo, Japan).
Reagents BSA, DMEM medium, SB203580, and Hoechst 33258 were purchased from Sigma Chemical Co (St Louis, MO, USA). phospho-p38 MAPK monoclonal antibody, horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody, and a Phototope-HRP Western Detection kit were purchased from New England Biolabs Inc (New England Biolabs, Ipswich, MA, USA).
Statistical analysis Values were expressed as mean±SD and assessed by one-way ANOVA and Student’s t-test. Values of P<0.05 were considered to be statistically significant.
Results
Cell viability Low doses of aspirin protected BAEC from apoptosis induced by H2O2. Co-incubation of aspirin 1×10-10–1×10-8 mol/L reversed the decrease of BAEC viability induced by H2O2 at 200 µmol/L. Interestingly, aspirin at 1×10-9 mol/L exhibited a stronger protective effect than 1×10-10 and 1×10-8 mol/L (Figure 1).
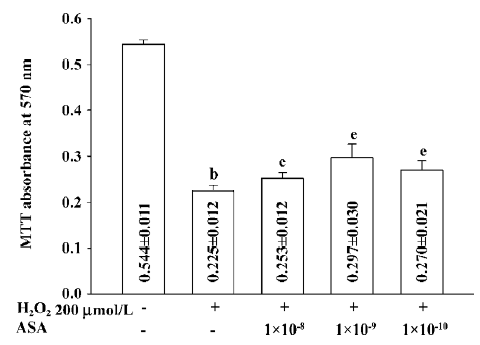
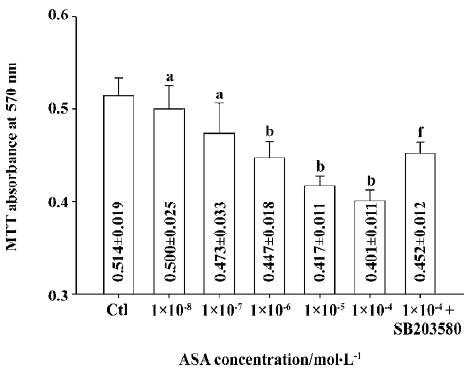
Unexpectedly, at relatively high concentrations, aspirin from 1×10-7 mol/L to 1×10-4 mol/L, showed no protection effect against the apoptosis of BAEC (data not shown), and it by itself decreased the viability of BAEC. After incubation with 1×10-4 mol/L aspirin for 24 h, cell viability decreased to 78.0% of the control (absorbance: 0.401±0.011 vs 0.514±0.019 of the control, P<0.01). SB203580 (10 µmol/L), a specific p38 MAPK inhibitor, markedly reduced the toxic effect of aspirin and increased cell viability by about 12.7% (absorbance: 0.452±0.012 vs 0.401±0.011 of 1×10-4 mol/L aspirin, P<0.01; Figure 2).
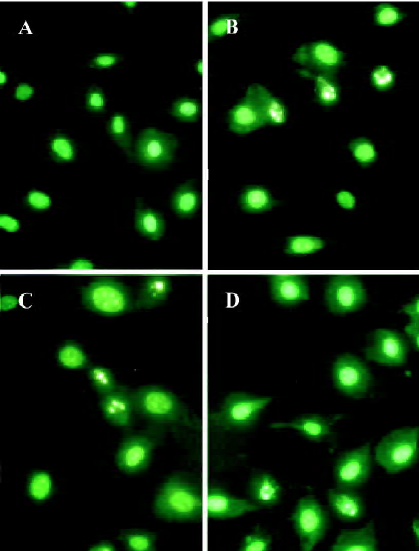
Morphologic changes After 24 h incubation of the indicated treatments, BAEC showed typical morphologic changes of apoptosis, such as reduction of cell volume and condensation and fragmentation of chromosomes; normal cells showed uniformed nuclei (Figure 3).
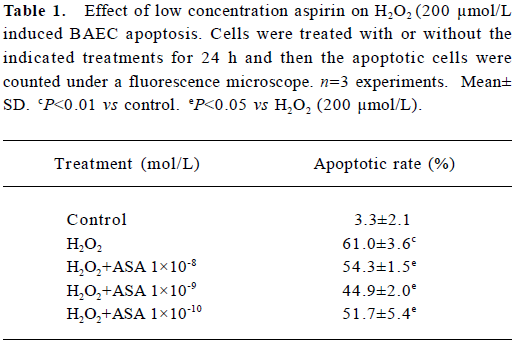
Quantification of apoptosis To further determine that the viability change of BAEC was mainly caused by apoptosis rather than necrosis, the apoptotic rate was directly measured under fluorescence microscope as described in Materials and methods.
At low concentration, aspirin from 1×10-10 mol/L to 1×10-8 mol/L reduced the apoptotic rate induced by 200 µmol/L H2O2 (Table 1). Incubation with aspirin (1×10-4 mol/L) for 24 h increased the apoptotic rate of BAEC to 26.0%±1.8%, while pretreatment with SB203580 reduced the apoptotic rate to 13.9%±2.0% (Table 2). These results were consistent with the results in the cell viability assay.
Full table
Full table
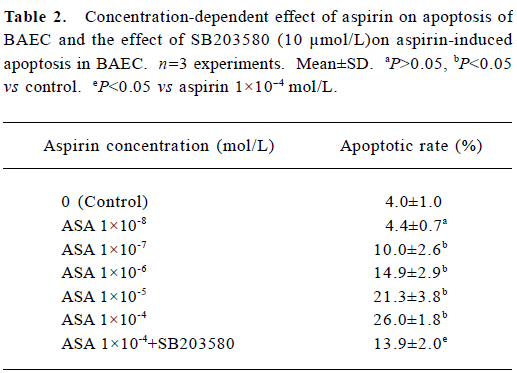
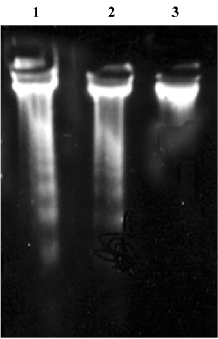
DNA electrophoresis Following analyses with 1.5% agarose gel, DNA isolated from BAEC treated with 200 µmol/L H2O2 for 24 h showed a typical DNA ladder which represents integer multiples of the internucleosomal DNA length (about 180–200 base pair). Aspirin at 1×10-9 mol/L greatly reduced DNA fragmentation (Figure 4). Incubation with aspirin (1× 10-4 mol/L) for 24 h also induced a typical DNA ladder which was reversed by 10 µmol/L SB203580, a specific inhibitor of p38 MAPK (Figure 5).
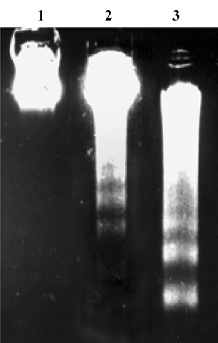
Phospho-p38 MAPK expression We previously showed that H2O2 activated p38 MAPK in BAEC[16]. In this study, aspirin from 1×10-10 to 1×10-8 mol/L significantly decreased p38 MAPK phosphorylation induced by 200 µmol/L H2O2 (Figure 6). Interestingly, aspirin at 1×10-9 mol/L exhibited the strongest effect, which paralleled well with its anti-apoptotic effect.
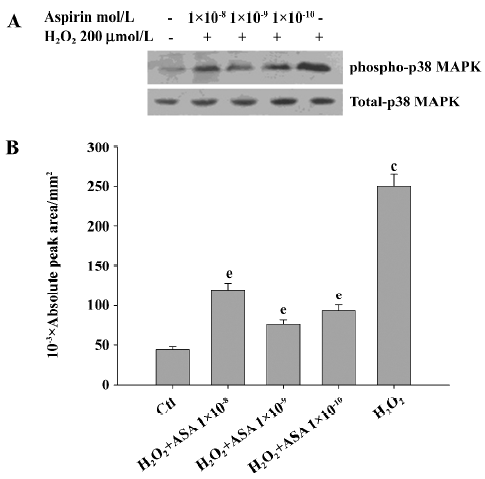
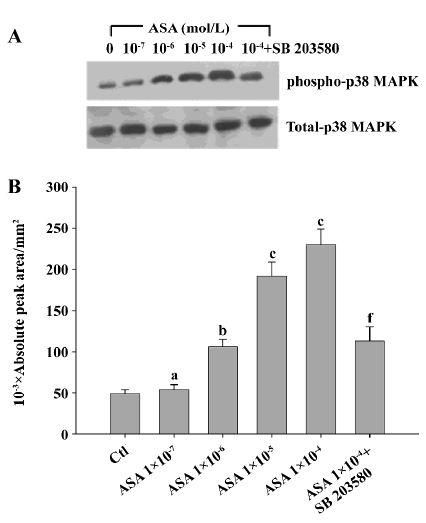
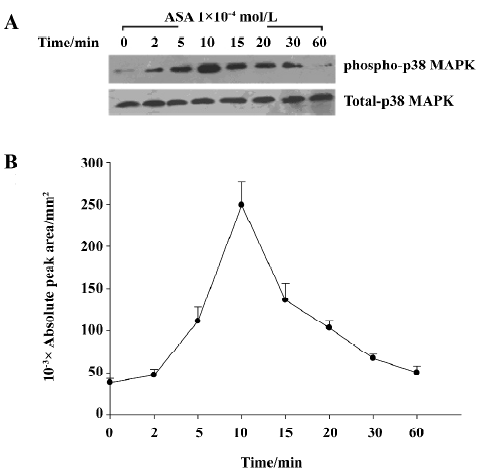
Aspirin from 1×10-7 mol/L to 1×10-4 mol/L dose-dependently increased the phosphorylation of p38 MAPK in BAEC, while SB203580 at 10 µmol/L blocked such change (Figure 7). Aspirin (1×10-4 mol/L) stimulated the phosphorylation of phospho-p38 MAPK in a time-dependent manner with a peak effect at 10 min, returning back to baseline at 1 h (Figure 8).
Discussion
Aspirin was introduced as an anti-inflammatory and analgesic drug in 1892, and the inhibition of COX has been thought to be the major mechanism mediating this effect. During the past decades, novel actions of aspirin have been discovered, which therefore largely expands the clinical use of aspirin. Experimental evidence has shown that aspirin can protect endothelial cells from oxidant damage through the NO–cGMP pathway, which provides a base for its use as a cardiovascular protective drug to reduce the occurrence of heart attack and stroke[7]. Aspirin has also been found to promote the apoptosis of tumor cells, and can be used to protect against the development of colon cancer and other digestive system cancers[5,17,18]. In the present study, we demonstrate that aspirin exhibits a biphasic effect on the apoptosis of bovine endothelial cells; at low concentrations, it protects BAEC from apoptosis induced by H2O2, while at relatively high concentrations, aspirin by itself induces apoptosis in BAEC. The endothelium protective effect of aspirin observed at low concentrations is consistent with its clinical practice that only a low dose of aspirin is required for its preventive action on cardiovascular diseases. The observed apoptotic effects on endothelial cells at high concentrations sheds new light on its clinical use as a preventive drug for the carcinogenesis of digestive tract cancers because apoptosis of endothelial cells is a very effective way of blocking angiogenesis, a pivotal biological aspect in tumor development[10]. Our findings may also explain that the preventive effect of aspirin on carcinogenesis is mostly limited in the digestive tract, because a relatively high local concentration of aspirin may only be reached in the digestive tract.
We next investigated the mechanisms mediating the biphasic effect of aspirin on BAEC apoptosis. Aspirin has been shown to induce apoptosis of oesophageal cancer cells and MCF-7 cells in a COX-dependent and independent manner, respectively[5,19]. However, aspirin has also been shown to inhibit endothelial cell apoptosis via its antioxidant effects[20]. MAPK signal cascades have been extensively studied in the regulation of cell proliferation and apoptosis. There are mainly 3 subfamilies, of which, p44/42 MAPK is activated by growth factors and considered to be related to cell growth and survival. In contrast, p38 MAPK and stress-activated protein kinase (SAPK)/JNK are usually activated by stress and pro-inflammatory cytokines, and are closely associated with apoptosis. Recent studies indicate that MAPK signal cascades mediate the effect of aspirin on the proliferation or apoptosis of different cell types[12,21,22]. In this study, we found that aspirin at low concentrations inhibited H2O2-induced apoptosis as well as the phosphorylation of p38 MAPK in BAEC. In contrast, at relatively high concentrations, aspirin directly triggered apoptosis and p38 MAPK activation in BAEC. By using a specific p38 MAPK inhibitor, SB203580, we provide convincing evidence that p38 MAPK is involved in mediating the biphasic effect of aspirin on endothelial cells. Previous studies reported in our lab or by others have shown that SB203580 blocks p38 MAPK-mediated apoptosis in a stimulus-dependent manner[16,23]. Recent studies have shown that there are at least 4 different subfamily members of p38 MAPK: α, β, γ, and δ; SB203580 can only inhibit member α and β[24]. Our data suggest that the member α and/or β of p38 MAPK are involved in mediating the effects of aspirin on the apoptosis of BAEC. However, we can not exclude the involvement of the other 2 p38 MAPK sub-family members. Further investigation is required to clarify this.
In conclusion, aspirin has a biphasic effect on the apoptosis of BAEC. It can induce apoptosis at high concentrations and inhibit apoptosis at low concentrations. p38 MAPK is an important signal molecule mediating the effects of aspirin on endothelial cell apoptosis.
References
- Rodondi N, Cornuz J, Bauer DC. Aspirin for the primary prevention of cardiovascular disease: a comprehensive review. Compr Ther 2005;31:186-93.
- Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology 2002;59:880-6.
- Gill S, Sinicrope FA. Colorectal cancer prevention: is an ounce of prevention worth a pound of cure? Semin Oncol 2005;32:24-34.
- Schror K. Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost 1997;23:349-56.
- Liu JF, Jamieson GG, Drew PA, Zhu GJ, Zhang SW, Zhu TN, et al. Aspirin induces apoptosis in oesophageal cancer cells by inhibiting the pathway of NF-kappaB downstream regulation of cyclooxygenase-2. ANZ J Surg 2005;75:1011-6.
- Czyz M, Watala C. Aspirin-the prodigious panacea? Molecular mechanisms of the action of acetylsalicylic acid in the organism. Postepy Hig Med Dosw 2005;59:105-15.
- Grosser N, Schroder H. Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cGMP pathway. Arterioscler Thromb Vasc Biol 2003;23:1345-51.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26:239-57.
- Cullen P, Rauterberg J, Lorkowski S. The pathogenesis of atherosclerosis. Handb Exp Pharmacol 2005;170:3-70.
- Cao Y. Tumor angiogenesis and therapy. Biomed Pharmacother 2005;59 Suppl 2:S340-3.
- Sumbayev VV, Yasinska IM. Regulation of MAP kinase-dependent apoptotic pathway: implication of reactive oxygen and nitrogen species. Arch Biochem Biophys 2005;436:406-12.
- Mifflin RC, Saada JI, Di Mari JF, Valentich JD, Adegboyega PA, Powell DW. Aspirin-mediated COX-2 transcript stabilization via sustained p38 activation in human intestinal myofibroblasts. Mol Pharmacol 2004;65:470-8.
- Abiru S, Nakao K, Ichikawa T, Migita K, Shigeno M, Sakamoto M, et al. Aspirin and NS-398 inhibit hepatocyte growth factor-induced invasiveness of human hepatoma cells. Hepatology 2002;35:1117-24.
- Mehta JL, Chen J, Yu F, Li DY. Aspirin inhibits ox-LDL-mediated LOX-1 expression and metalloproteinase-1 in human coronary endothelial cells. Cardiovasc Res 2004;64:243-9.
- Han YF, Su CJ, Ou BR. The effect and mechanism of felodipine and valsartan on a novel salt-sensitive hypertensive rat induced by sensory denervation. Chin J Cardiol 2005;33:255-9. Chinese..
- Liu WL, Guo X, Guo ZG. Effects of p38 and p42/p44 CCDPK signaling on H2O2-induced apoptosis in bovine aortic endothelial cells. Acta Pharmacol Sin 2000;21:991-6.
- Ashktorab H, Dawkins FW, Mohamed R, Larbi D, Smoot DT. Apoptosis induced by aspirin and 5-fluorouracil in human colonic adenocarcinoma cells. Dig Dis Sci 2005;50:1025-32.
- Benamouzig R, Uzzan B, Little J, Chaussade S. Low dose aspirin, COX-inhibition and chemoprevention of colorectal cancer. Curr Top Med Chem 2005;5:493-503.
- Teh SH, Hill AK, Foley DA, McDermott EW, O’Higgins NJ, Young LS. COX inhibitors modulate bFGF-induced cell survival in MCF-7 breast cancer cells. J Cell Biochem 2004;91:796-807.
- Costanzo A, Moretti F, Burgio VL, Bravi C, Guido F, Levrero M, et al. Endothelial activation by angiotensin II through NFkappaB and p38 pathways: Involvement of NF-kappaB-inducible kinase (NIK), free oxygen radicals, and selective inhibition by aspirin. J Cell Physiol 2003;195:402-10.
- Vartiainen N, Goldsteins G, Keksa-Goldsteine V, Chan PH, Koistinaho J. Aspirin inhibits p44/42 mitogen-activated protein kinase and is protective against hypoxia/reoxygenation neuronal damage. Stroke 2003;34:752-7.
- Ordan O, Rotem R, Jaspers I, Flescher E. Stress-responsive JNK mitogen-activated protein kinase mediates aspirin-induced suppression of B16 melanoma cellular proliferation. Br J Pharmacol 2003;138:1156-62.
- Liu WL, Guo X, Chen QQ, Guo ZG. Opposing effect of p38 CCDPK and p44/42 CCDPK signaling on TNF-alpha-induced apoptosis in bovine aortic endothelial cells. Acta Pharmacol Sin 2001;22:405-10.
- Alonso G, Ambrosino C, Jones M, Nebreda AR. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J Biol Chem 2000;275:40641-8.
