Stereoselectivity in metabolic 3-reduction of tibolone in healthy Chinese female volunteers
Introduction
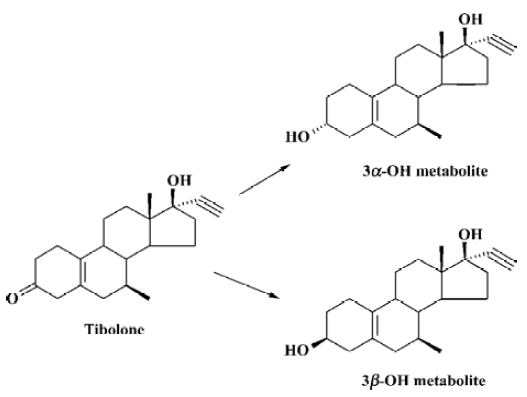
Tibolone, [7α,17α-7-methyl-17-hydroxyl-19-norpregn-5(10)-en-20-yn-3-one], also called 7-methyl-norethynodrel (MN), is a synthetic steroid (Figure 1) used in hormone replacement therapy (HRT) for postmenopausal women. Clinical data indicates that tibolone can produce the hormonal effects needed to treat climacteric symptoms and to prevent the long-term effects of menopause without stimulating the breast or endometrial tissues[1–3]. After administration to women, the compound is quickly metabolized into a 3α-hydroxyl metabolite (3α-hydroxyl-7-methyl- norethynodrel; 3α-HMN) and a 3β-hydroxyl metabolite (3β-hydroxyl-7-methyl- norethynodrel; 3β-HMN) by the enzymes 3α/β-hydroxyste-roid dehydrogenase in the intestine and the liver[4,5]. Tibolone itself has weak binding affinities to human estrogen, progesterone and androgen receptors, whereas the two 3-hydroxyl metabolites bind solely to the estrogen receptor, and 3α-HMN is approximately 3 times as effective as 3β-HMN[6]. The clinical merits of tibolone are thought to be a result of the tissue-specific activity of the drug and its main metabolites[7,8].
The pharmacokinetics of tibolone in female patients have been studied recently, and the plasma concentrations of 3α-HMN and 3β-HMN were determined by using a gas chromatography-mass spectrometry (GC-MS) method[9]. However, the pharmacokinetics of tibolone metabolism in a Chinese human population have not been studied. The present study was undertaken to investigate stereoselectivity in the pharmacokinetics of tibolone metabolism in healthy Chinese female subjects using a validated liquid chromatography-mass spectrometry (LC-MS) method.
Materials and methods
Subjects Twenty healthy Chinese female subjects ranging in age from 30 to 40 years (36.4±3.6 years), in weight from 47 to 67 kg (56.1±5.8 kg), and in height from 143 to 170 cm (157.1±6.4 cm) were enrolled in the study. All subjects were in good health and underwent a pre-enrolment screening that included ascertaining the subjects’ medical history, a physical examination, an electrocardiogram (ECG), laboratory tests and urinalysis. No medication was used by the subjects for at least 2 weeks before the study and alcohol was forbidden within the 72 h before drug administration. Approval was obtained from the Institutional Review Board of the Obstetrics and Gynecology Hospital, Medical Center of Fudan University, Shanghai, and all subjects gave written informed consent.
Clinical protocol After an overnight fast of at least 10 h, subjects took one tablet of Livial, which contained 2.5 mg tibolone, with 200 mL water and continued fasting for 2 h. Blood samples obtained from an antecubital vein prior to treatment and at 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 6.0, 8.0, 12.0, and 18.0 h after treatment were placed in heparinized tubes. The samples were immediately centrifuged at 3000×g for 15 min, and the plasma was separated and frozen at -20 °C until analysis.
LC-MS analysis of the metabolites in plasma Plasma concentrations of 3α-HMN and 3β-HMN were determined by using a LC-MS method described elsewhere[10]. Plasma samples were derivatized with p-toluenesulfonyl isocyanate after extraction with ethyl acetate. Separation of the two derivatized 3-hydroxyl metabolites was carried out on a Diamonsil C18 column with a linear gradient elution of mixtures of methanol and ammonia acetate aqueous solution. The analytes were detected with a mass spectrometry detector in the negative selected ion monitoring (SIM) mode.
The limit of quantitation for both 3α-HMN and 3β-HMN was 100 ng/L, and the accuracy was 91.6%–116% for 3β-HMN and 90.4%–104% for 3β-HMN. The inter-day and intra-day coefficients of variation for 3α-HMN and 3β-HMN were less than 13.8% and 11.3%, respectively, over the concentration range 0.1–30 µg/L.
Pharmacokinetic analysis Pharmacokinetic parameters were estimated by using non-compartmental analysis of curves of 3α/β-HMN isomer plasma concentrations versus time. Maximum plasma concentrations (Cmax) and the corresponding times (Tmax) were read as the coordinates of the highest raw data point for each volunteer. The area under the plasma concentration versus time curve (AUC0–t) was estimated by using the linear trapezoidal rule up to the last measurable time. AUC0–∞ was obtained by adding the part of the area extrapolated to infinity (last measurable concentration/ke) to AUC0-t, where ke is the slope of the linear regression analysis of natural log concentrations against time. The elimination half-life (T½) was estimated from the plasma data by using the equation T½=0.693/ke. The values were expressed as mean±SD. Differences between 3α-HMN and 3β-HMN in the pharmacokinetic data were evaluated statistically by using the independent-samples t-test. P<0.05 was considered statistically significant.
Results
Plasma drug concentration-time curves After subjects were given tibolone, the mean plasma concentrations of 3α-HMN and 3β-HMN were all below 10 ng/mL, and the mean plasma concentrations of 3α-HMN were much greater than those of 3β-HMN (Figure 2). The last time point for which all subjects had measurable concentrations of 3α-HMN and 3β-HMN was 18 h.
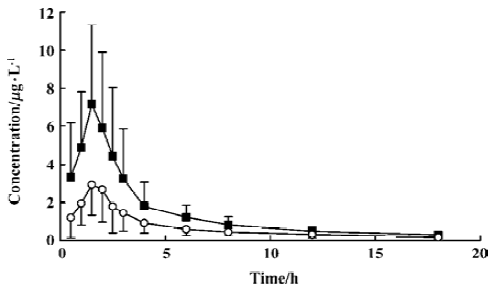
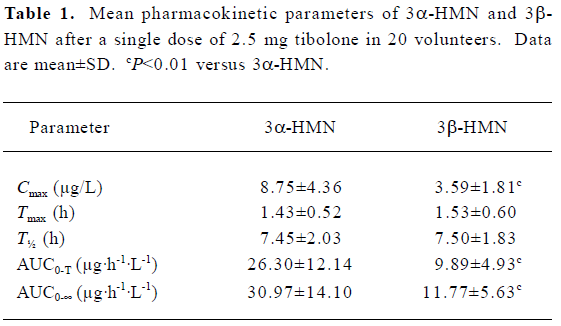
Pharmacokinetic parameters of the metabolites After subjects were given tibolone at a dose of 2.5 mg, Cmax values for 3α-HMN and 3β-HMN were estimated to be 8.75 and 3.59 µg/L, and the mean AUC0-t values were 26.30 and 9.89 µg·h-1· L-1, respectively.
There was no significant difference in Tmax and T½ between 3α-HMN and 3β-HMN (P>0.05, Table 1). After subjects were given tibolone, the two metabolites both reached peak concentrations in the blood at 1.5 h, and T½ values for 3α-HMN and 3β-HMN were 7.45±2.03 h and 7.50±1.83 h, respectively.
Full table
Discussion
The mean pharmacokinetic parameters of 3β-HMN were similar to those found by Timmer et al[9], whereas the peak plasma concentration of 3α-HMN in our study (8.75±4.36 µg/L) was much lower than that reported elsewhere for early (14.6±5.4 µg/L)[9] or late postmenopausal women (16.7±6.6 µg/L)[9]. Moreover, the AUC0–∞ of 3α-HMN in the present study (30.97±14.10 µg·h-1·L-1) was also lower than that reported elsewhere for early (49.6±14.6 µg·h-1·L-1) or late postmenopausal women (62.6±17.3 µg·h-1·L-1)[9].
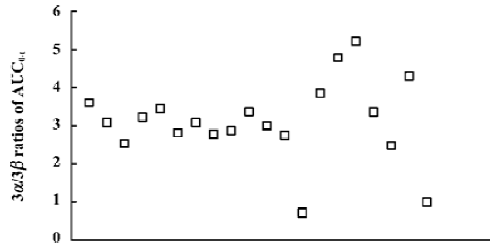
There was a significant difference between the two 3-hydroxyl metabolites in all mean pharmacokinetic parameters except for Tmax and T½ (Table 1). The mean Cmax and AUC0–t values of 3β-HMN were approximately 59% and 62% lower than that of 3α-HMN, respectively. Mean 3α/3β stereoisomer ratios for plasma concentrations of the two metabolites in 20 subjects ranged from 1.5 to 3.0 after administration (Figure 3). These results indicate that a stereoselective difference exists in the 3-hydroxylization metabolism of tibolone.
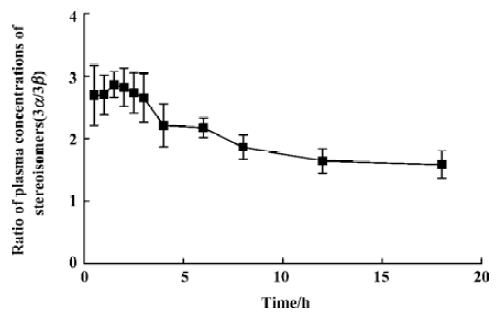
Although a 3α-hydroxylization intensive metabolism was found for the metabolism pharmacokinetics of tibolone in the 20 subjects as a whole (with a mean 3α/3β AUC0–T ratio of 3.11), two 3α-hydroxyl-poor metabolizers were found in the present study (with 3α/3β AUC0–t ratios of 0.71 and 0.99; Figure 4). Differences in the 3α/β-hydroxylization metabolism of tibolone, as well as desogestrel, by different species of animals have been confirmed by Verhoeven et al[11,12]: both drugs had mainly 3α-hydroxyl metabolites in rats, but 3β-hydroxyl metabolites in dogs. Because 3α-hydroxysteroid dehydrogenase and 3β-hydroxysteroids dehydrogenase reduce 3-keto steroids, polymorphism for 3α/β-hydroxylization metabolism can be explained by individual differences in 3α-hydroxysteroid dehydrogenase activity and 3β-hydro-xysteroid dehydrogenase activity.
Acknowledgement
The authors express their thanks to Prof Shao-fen ZHANG (Department of Gynecology, The Obstetrics and Gynecology Hospital, Medical Center of Fudan University, Shanghai) and her group for their help in subject selection and drug administration, and assistance in the medical ward.
References
- Paola A, Raffaele DM, Ettore Z. Tibolone: a review. Maturitas 1998;30:295-305.
- Valdivial I, Ortega D. Mammographic density in postmenopausal women treated with tibolone, eatriol or conventional hormone replacement therapy. Clin Drug Invest 2000;20:101-7.
- Lundstrom E, Christow A, Kersemaekers W, Svane G, Azavedo E, Soderqvist G, et al. Effects of tibolone and a continuous combined HRT regimen on mammographic breast density. Am J Obstet Gynecol 2002;186:717-22.
- Sandker GW, Vos RME, Delbressine LPC, Slooff MJ, Meijer DK, Groothuis GM. Metabolism of three pharmacologically active drugs in isolated human and rat hepatocytes: analysis of interspecies variability and comparison with metabolism in vivo. Xenobiotica 1994;24:143-55.
- Vos REM, Krebbers SFM, Verhoeven CHJ, Delbressine LPC. The in vivo human metabolism of tibolone. Drug Metab Dispos 2002;30:106-12.
- Schoonen WG, Deckers GH, de Gooyer ME, de Ries R, Kloosterboer HJ. Hormonal property of norethisterone, 7α-methyl-norethisterone and their derivative. J Steroid Biochem Mol Biol 2000;74:213-22.
- Palacios S. Tibolone: what does tissue specific activity mean? Maturitas 2001;37:159-65.
- Kloosterboer HJ. Tibolone: a steroid with a tissue-specific mode of action. J Steroid Biochem & Mol Biol 2001;76:231-8.
- Timmer CJ, Verheul AM, Doorstam DP. Pharmacokinetics of tibolone in early and late postmenopausal women. Br J Clin Pharmacol 2002;54:101-6.
- Zuo M, Gao MJ, Liu Z, Cai L, Duan GL. p-Toluenesulfonyl isocyanate as a novel derivatization reagent to enhance the electrospray ionization and its application in the determination of two stereo isomers of 3-hydroxyl-7-methyl-norethynodrel in plasma. J Chromatogr B 2005;814:331-7.
- Verhoeven CHJ, Vos REM, Delbressine LPC. The in vivo metabolism of tibolone in animal species. Eur J Drug Metab Pharmacokinet 2002;27:1-10.
- Verhoeven CHJ, Krebbers SFM, Wagenaars GN, Vos RME. In vitro and in vivo metabolism of desgestrel in several species. Drug Metab Dispos 1998;26:927-36.