Evaluation of 2 celecoxib derivatives: analgesic effect and selectivity to cyclooxygenase-2/11
Introduction
Humans have been using non-steroidal anti-inflammatory drugs (NSAIDs) for more than 3500 years. The first real progress in our understanding of the mechanism of the action of NSAIDs occurred 30 years ago, when it was revealed that these chemically varied drugs reduced the formation of prostaglandins. This ability is associated with the inhibition of cyclooxygenase (COX), which converts arachidonic acid to its precursor prostaglandin (PG) H2. Two isoforms of COX have been identified. The constitutively expressed COX-1 is the housekeeping enzyme mainly responsible for the physiological activities of prostaglandins, whereas COX-2, whose expression is mainly induced under inflammatory conditions, is responsible for pathological prostaglandins that produce pain and fever[1]. On the basis of these observations, COX-2-selective drugs such as celecoxib were rapidly developed and became one of the most commercially successful classes of drugs[2].
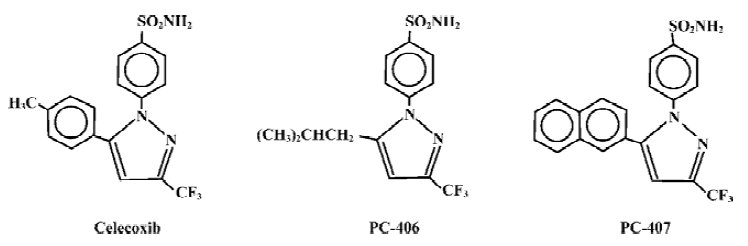
We synthesized 13 pyrazole derivatives based on the chemical structure of celecoxib[3,4], including PC-406 and PC-407, which are derivatives of celecoxib created by changing the group at the 5 position in the pyrazole ring. For PC-406, the phenyl structure at the 5 position is replaced by an isopropyl group; for PC-407, it is replaced by a naphthyl group (Figure 1). The chemical structures of these derivatives have been confirmed by infrared absorption spectrum, ultra-violet absorption spectrum, 1H nuclear magnetic resonance, 13C nuclear magnetic resonance and mass spectrum analysis. The purity values of PC-406 and PC-407 were found to be 99.5% and 97.5%, respectively, by high performance liquid chromatography. Using the mouse ear edema model, PC-406 and PC-407 were found to have more potential activity than celecoxib in anti-inflammation. PC-406 and PC-407 were also observed to have fewer gastrointestinal side effects. In the present study, we tried to evaluate the analgesic effects and inhibitory effects on COX-1/COX-2 of candidate compounds.
Materials and methods
Animals BALB/c mice obtained from the Experimental Animal Center of the Fourth Military Medical University were used. Half of the mice were males and half were females. All the animals weighed 18–22 g. Animals were housed in colonial stock following arrival. Food and water were available ad libitum. Temperature and humidity of the environment were controlled (23±1°C and 50.0%±1.3%, respectively), and a 12 h day/night cycle was used. All the experiments were carried out during the light phase. The experimental design was approved by the relevant ethics board of the institution in which the study was carried out.
Drugs and reagents PC-406 and PC-407 were synthesized by our group. The celecoxib was a gift from Pharmacia (Rockville, MD, USA). Lipopolysaccharide (LPS) and calcimycin were purchased from Sigma (St Louis, USA). The Brewer thioglycollate medium was from (Difco Company, Detroit, USA). Newborn calf serum and RPMI-1640 were from (Hyclone, Logan, USA). The 6-keto-PGF1α and PGE2 radioimmunoassay (RIA) kits were from Chemclin Biotech (Beijing, China). For the analgesic effect assays, all the test compounds were dissolved in 3% Tween-80 and administered intragastrically in a volume of 0.3 mL. For the selectivity evaluation, all the tested samples were prepared in stock solution (0.01 mol/L) with Me2SO and stored at -20 °C. Before use, the stock solution was diluted to a series of concentrations in RPMI-1640.
Antinociceptive assays Four antinociceptive assays were used: the writhing test, hot tail-flick test, hot plate test, and formalin test. Mice were fasted for 24 h with water given ad libitum, maintained at room temperature and were divided into 4 groups (n=6): control group, CEL group, PC-406 group, and PC-407 group, which received pretreatment with 3% Tween-80, celecoxib, PC-406, and PC-407, respectively. The 2nd, 3rd, and 4th groups were further divided into 3 subgroups (A,B,C), which received test compounds at 100, 50, and 25 mg/kg, respectively. Celecoxib was used as a positive control.
Writhing test All the pretreatments were carried out 1 h prior to intraperitoneal injection of 0.1 mL/10 g of 0.6% acetic acid, which caused a typical writhing response. The number of writhing responses was counted for 10 min by observers who were blinded to the treatment. The antinociceptive effects of drugs were measured by calculating the mean reduction in the number of abdominal constrictions for each drug, as compared to that produced by the Tween-80 vehicle.
Hot tail-flick test The antinociceptive effects of the test substances were determined by the hot tail-flick method as described by Sewell and Spencer[5]. The tails of mice (1–2 cm) were immersed in warm water kept constant at 53 °C, and the reaction time was measured as the time taken for the mice to deflect their tails. The first reading was ignored, and the reaction time was taken as the mean of the next 2 readings. The latent period until the tail-flick response was taken as the index of antinociception and was determined before and 1 h after the administration of drugs. The maximum reaction time was fixed at 3 s. The analgesia percentage (AP) was calculated as: AP=(ttest reaction-tcontrol reaction)/(3-tcontrol reaction).
Hot plate test The method used has been described by Baker et al[6]. A metal hot plate was heated to a constant temperature. Behavioral measurements were taken at 55±0.5 °C. The temperature of the plate was monitored at all times. To confine the animals to a certain observation area, a colorless acrylic cylinder of 20 cm diameter was placed on the hot plate. After each measurement the plate was wiped with a damp cloth to remove traces of urine and feces. Latency for the animal to lick its hindpaw was measured before and 1 h after pretreatment. The offset time was set at 30 s. AP was calculated as: AP=(ttest reaction-tcontrol reaction)/(30-tcontrol reaction).
Formalin test Formalin solution (5%, 50 mL) was injected subplantarly into the left hindpaws of the mice using a microsyringe with a 26-gauge needle. Animals were then placed in a Plexiglas chamber and observed for nociceptive behavior for 1 h. The amount and duration of licking or flinching were recorded at 5 min intervals over the next 60 min. The sum of the amount and duration of licking or flinching in the early phase (0–10 min, phase 1) and late phase (20–60 min, phase 2) were calculated, respectively. To calculate the ED50, the decrease in the summed amount of licking or flinching during the whole observation period as compared with the control group was calculated.
Cell culture Adherent macrophages were harvested from the peritoneal cells of male mice 3 d after the intraperitoneal injection of Brewer thioglycollate medium (50 mL/kg). Peritoneal cells obtained from 2–3 mice were mixed and cultured in RPMI-1640 supplemented with 5% new-born calf serum. After settlement for 2 h, non-adherent cells were washed with cold phosphate-buffered saline (PBS). Then, macrophages were seeded in 96-well cell culture clusters (Gibco, USA) at a cell density of 1×109/L. Almost all the adherent cells were macrophages as assessed by Giemsa staining. Cell viability was examined by Trypan blue dye exclusion. All the incubation procedures were performed with 5% CO2 in humidified air at 37 °C.
COX-1 assay Macrophages were incubated with test compounds at different concentrations or with the solvent (Me2SO) for 1 h and were stimulated with calcimycin (1 μmol/L) for 1 h. The same volume of RPMI-1640 was added for the control groups. The amount of 6-keto-PGF1α in the supernatant was measured by RIA, using the manufacturer’s instructions. The inhibitory ratio (IR) was calculated as IR = (Ccalcimycin-Ctest compound)/(Ccalcimycin-Ccontrol), where C is the concentration of 6-keto-PGF1α in the supernatants of the calcimycin, test compound or control groups.
COX-2 assay Macrophages were incubated with test compounds at different concentrations or with the solvent (Me2SO) for 1 h and were stimulated with LPS (1 mg/L) for 9 h. The same volume of RPMI-1640 was added for the control groups. The amount of PGE2 in the supernatant was measured by RIA, using the manufacturer’s instructions. The IR was calculated as IR=(CLPS -Ctest compound)/(CLPS-Ccontrol), where C is the concentration of PGE2 in the supernatants of the LPS, test compound or control groups.
Statistics Data are presented as mean±SD of n observa-tions. The 95% confidence limits of the ED50 and IC50 values were also calculated. Comparisons between the groups were carried out by using one-way analysis of variance (ANOVA), followed by the least significant difference multiple comparisons test. A P-value of 0.05 was considered statistically significant. Dose-inhibitory effect curves were fitted by using sigmoidal dose-response curves (variable slope)(inhibitory effect on COX) or linear regression (analgesic effect) using Graphpad Prism (version 4.00, GraphPad Software Inc, San Diego, USA).
Results
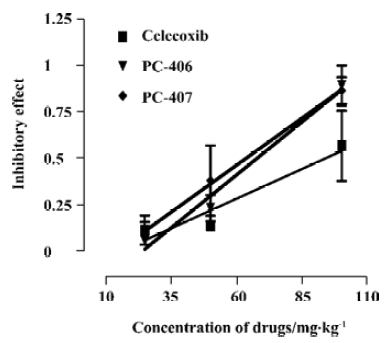
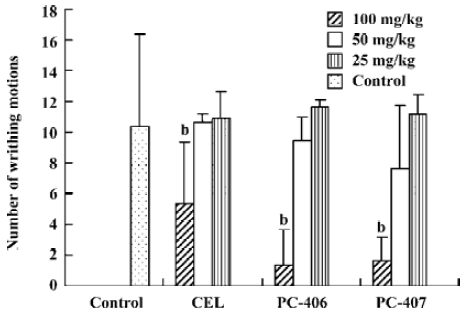
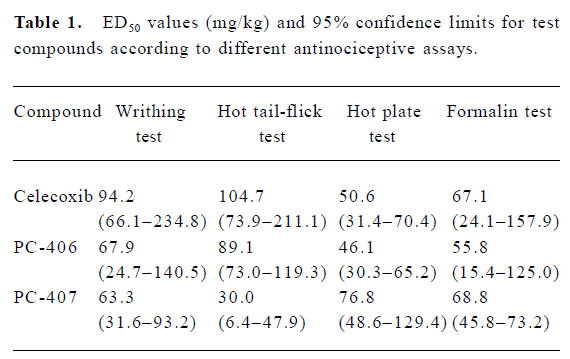
Acetic acid-induced writhing Intraperitoneally injected acetic acid produced abdominal constrictions, which were characterized by a stretching response. The mean number of writhing motions observed in the animals of the control group over 10 min was 10.33±6.03. All the 3 test compounds at a high dose (100 mg/kg) significantly (P<0.05) reduced the number of writhing motions to 5.3±4.0, 1.3±2.3, and 1.7±1.5, respectively. However, test compounds at low doses (50 or 25 mg/kg) did not significantly reduce the number of writhing motions induced by acetic acid (Figure 2). The ED50 values of celecoxib, PC-406 and PC-407 for acetic acid-induced writhing were 94.2, 67.9, and 63.3 mg/kg respectively (Figure 3, Table 1).
Full table
Hot tail-flick response The animals in the control group did not show any significant effects with respect to the latent period until tail-flick response. The antinociceptive effect of PC-406 at a high dose (100 mg/kg) was evident (P<0.05) and the duration of the tail-flick response increased from 0.87±0.27 to 1.92±0.15 s. PC-406 at lower doses (25 or 50 mg/kg) did not have any significant analgesic effect as measured by this assay. However, PC-407 at all doses produced significant antinociception (P<0.05, Figure 5). The ED50 values of celecoxib, PC-406, and PC-407 as assessed by the hot tail-flick assay were 104.7, 89.1, and 30.0 mg/kg, respectively (Figure 4, Table 1).
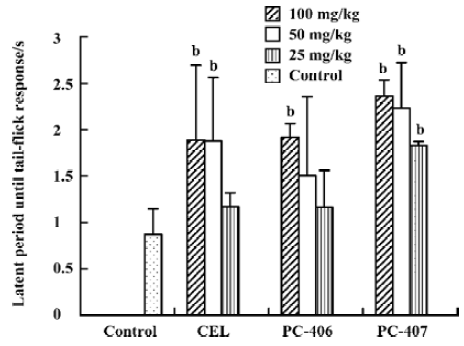
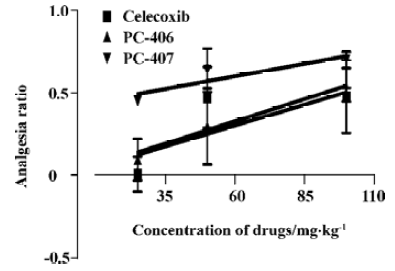
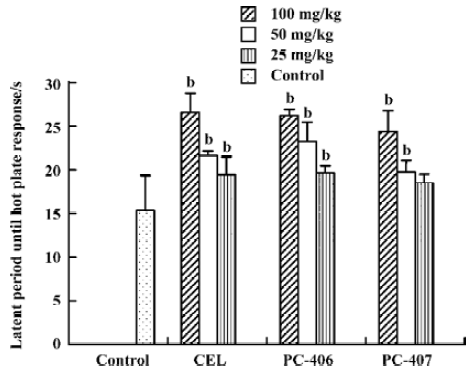
Hot plate response Animals in the control group did not show any significant effects with respect to the latent period until hot plate response. Both PC-406 and PC-407 produced a significant analgesic effect as measured by the hot plate response (P<0.05, Figure 5). The ED50 values of celecoxib, PC-406 and PC-407 as measured by hot plate response were 50.6, 46.1, and 76.8 mg/kg, respectively (Figure 6, Table 1) (Figure 7).
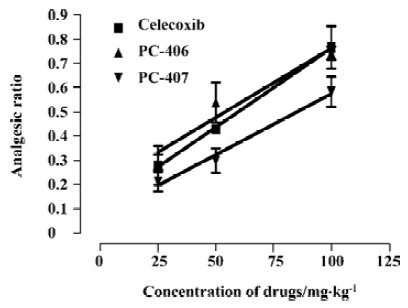
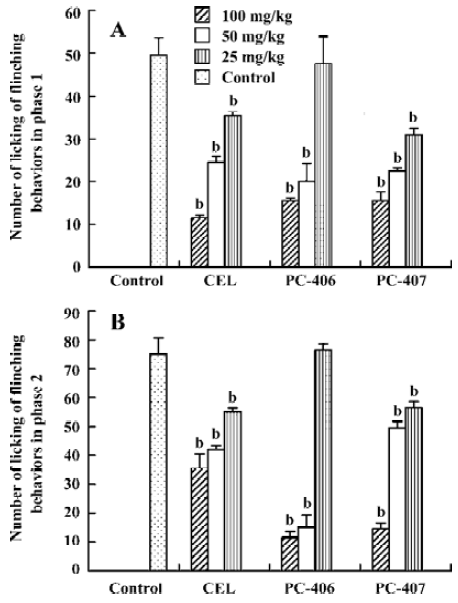
Formalin test All the mice exhibited elevating, shaking, licking or biting behaviors in the injected left hindpaw after subcutaneous injection of formalin into the plantar surface. These behaviors had 2 distinct phases: an early phase (phase 1), which appeared immediately after formalin injection and lasted 10 min, and a late phase (phase 2), which appeared about 20 min after injection and lasted 40 min. PC-406 and PC-407 (25–100 mg/kg) elicited a dose-dependent inhibitory effects on licking time, with a maximal reduction of approximately 85% of the control response (n=8). For animals treated with PC-406 or PC-407, the durations and amounts of flinching or licking were significantly decreased (P<0.05, Figures 8, 9). The ED50 values of celecoxib, PC-406 and PC-407 were 67.1, 55.8, and 68.8 mg/kg, respectively (Figure 10, Table 1).

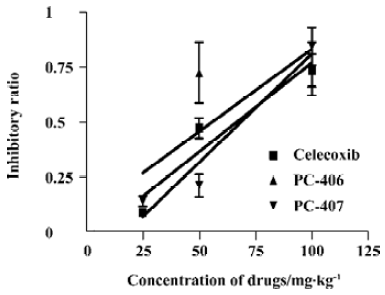
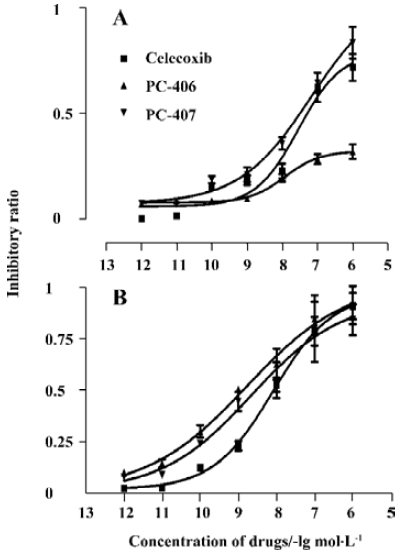
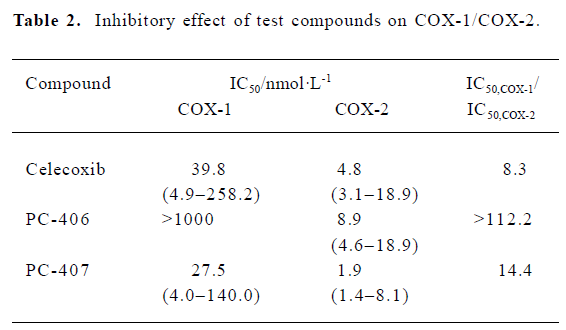
COX inhibitory effect The IC50 values of celecoxib and PC-407 for COX-1 were 39.8 nmol/L and 27.5 nmol/L, respectively. For PC-406, the IC50 value was more than 10 000 nmol/L (Figure 11A). The IC50 of test compounds for COX-2 were 4.78, 8.9, and 1.9 nmol/L, respectively (Figure 11B). The selectivity of the test compounds for COX-1/COX-2 was expressed as the value of IC50,COX-1/IC50,COX-2. PC-406 showed a 112.2-fold preference for COX-2 versus COX-1 (Table 2).
Full table
Discussion
The discovery and characterization of the COX-2 enzyme early in the 1990s led to many studies on selective inhibitors of this isoform. It was hypothesized that COX-2 selective inhibitors would exhibit similar clinical efficacy but reduced ulcerogenicity relative to traditional NSAIDs. Less than a decade after the discovery of COX-2, celecoxib and rofecoxib were launched, and were very effective analgesics and anti-inflammatory agents. Furthermore, in recent years, coxibs have been proved to have potential in the treatment of cancer and Alzheimer’s disease. All things considered, the prospects for COX-2 selective inhibitors are good. Based on the chemical structure of celecoxib, we synthesized PC-406 and PC-407, 2 pyrazole derivatives, by retaining benzenesulfona-mide, the necessary active component of the molecule. In the present study, their analgesic effects and selectivity for COX-2 were evaluated.
All the antinociceptive assays showed that derivatives of celecoxib made by substituting groups at the 5 position exhibited analgesic effects. However, the results from different assays were different. Four antinociceptive assays were used in our study. Among them, the hot plate and hot tail-flick tests test responses to thermal stimulations, whereas the writhing and formalin tests test responses to chemical stimulations. The pain pathways these tests focus on vary as well. The formalin and hotplate tests mainly focus on changes above the spinal cord level, whereas the tail flick and writhing tests emphasize changes at or under the spinal cord level. In the present study, the results of both the hot plate test and the formalin test show that PC-406 has the lowest ED50 value. However, the results of the writhing and tail flick test show that PC-407 has the lowest ED50 value. Thus we can infer that PC-406 exerts more effect on the pathways above the spinal cord level, whereas PC-407 exerts more effect on pathways peripherally.
Detailed data from the formalin test showed the same results. The formalin test is a well characterized and validated evaluation method in mice and rats. It is an accepted method for the rapid and easy screening of pharmacological targets in drug evaluation, because it can be performed in small animals, and the time course of the noxious stimulation is limited to 1 h. Subplantar injection of formalin results in flinching, licking or biting behavior during an early acute phase, which resembles acute pain, followed by a quiescent interphase, and then a second delayed phase representing chronic pain. The acute phase is believed to correspond to the peripheral pain pathways, whereas the second phase represents more central pain processing mechanisms. In our study, both phases of the formalin test were well delineated and could be quantified. Celecoxib and its 2 derivatives could significantly decrease both the duration and amount of pain behaviors during the formalin test (P<0.05). Celecoxib and PC-407 had more effect on behaviors in phase 1, whereas PC-406 affected behaviors in phase 2 more. That is, celecoxib and PC-407 affected the peripheral pain pathways more, whereas PC-406 affected the central pain pathways more. This is consistent with what we found in our analysis of different ED50 values.
The differences in the different analgesic effects of the test compounds are related to their different structures. So we subsequently investigated the relationships between the modifications of their chemical structures and their selectivity for COX-2. To evaluate the selectivity of compounds for COX-2, many models have been established[7–10]. Macrophages are known to release prostanoids in 2 kinetically distinct patterns: the immediate and delayed phases[11]. In the immediate phase, 6-keto-PGF1α and TXB2 are the major arachidonic acid metabolites. However, in the delayed phase, PGE2 is produced depending on induced COX-2. Macrophages can spontaneously secrete low levels of 6-keto-PGF1α and PGE2. Stimulation with calcimycin or LPS promotes a dramatic enhancement of prostanoid production. Shen et al developed a whole-cell assay based on murine peritoneal macrophages and demonstrated that it was appropriate for drug design-oriented in vitro assay[12]. This method was used in the present study.
The results of selectivity evaluation show that PC-407 and PC-406 provide stronger selectivity with respect to the inhibition of COX-2 than does celecoxib. PC-406 has the greatest selectivity. It might be the reason for their weak influence on stomach mucosa. The present study indicates that test compounds with a non-aryl group or an aryl group substituted at the 5 position in the pyrazole ring still retain their anti-inflammatory activity and their ability to inhibit COX-2 selectively. Furthermore, substitution at the 5 position with a non-aryl group might be more beneficial with respect to COX-2 selectivity than substitution with an aryl group.
It has been proved that COX-2 plays a more central role and is more highly expressed in the central nervous system than is COX-1, which is the basis of most of its therapeutic effects. The higher selectivity for COX-2 of PC-406 contributes to its central pain pathway effects. However, identification of the exact parts of the pain pathways that the 2 derivatives affect needs further investigation.
In conclusion, derivatives of celecoxib made by substituting with either a isopropyl or a naphthyl group at the 5 position in the pyrazole ring produced analgesic effects with respect to both thermal and chemical nociception. However, substitution with an isopropyl group improves the effect on the central pain pathway better, whereas substitution with a naphthyl group affects the peripheral pathway more. This is correlated with the altered selectivity for COX-2. Both derivatives had greater selectivity than celecoxib. However, substitution with an isopropyl group was more beneficial with respect to COX-2 selectivity than substitution with an naphthyl group.
References
- Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol 1997;24 Suppl 49:15-9.
- Lane JM. Anti-inflammatory medications: selective COX-2 inhibitors. J Am Acad Orthop Surg 2002;10:75-8.
- Zhang BL, Mei QB, He W, Zhou SY. Synthesis of celecoxib. Chin J New Drug 2002;11:859-61.
- Zhang BL, Mei QB, Zhou SY, He W, Chen CS. Quantitative structure-activity relationship of the 1, 5-diarylpyrazole class of cyclooxygenase-2 selective inhibitors. J Fourth Milit Med Univ 2003;24:1523-5.
- ewell RD, Spencer PS. Antinociceptive activity of narcotic agonist and partial agonist analgesics and other agents in the tail-immersion test in mice and rats. Neuropharmacology 1976;15:683-8.
- Baker AK, Hoffmann VL, Meert TF. Dextromethorphan and ketamine potentiate the antinociceptive effects of mu- but not delta- or kappa-opioid agonists in a mouse model of acute pain. Pharmacol Biochem Behav 2002;74:73-86.
- Gierse JK, Hauser SD, Creely DP, Koboldt C, Rangwala SH, Isakson PC, et al. Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase. Biochem J 1995;305:479-84.
- Brideau C, Kargman S, Liu S, Dallob AL, Ehrich EW, Rodger IW, et al. A human whole blood assay for clinical evaluation of biochemical efficacy of cyclooxygenase inhibitors. Inflamm Res 1996;45:68-74.
- Riendeau D, Percival MD, Boyce S, Brideau C, Charleson S, Cromlish W, et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol 1997;121:105-17.
- Lazer ES, Miao CK, Cywin CL, Sorcek R, Wong HC, Meng Z, et al. Effect of structural modification of enol-carboxamide-type nonsteroidal antiinflammatory drugs on COX-2/COX-1 selec-tivity. J Med Chem 1997;40:980-9.
- Naraba H, Murakami M, Matsumoto H, Shimbara S, Ueno A, Kudo I, et al. Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J Immunol 1998;160:2974-82.
- Shen F, Bai AP, Guo ZR, Cheng CF. Inhibitory effect of 3,4-diaryl-3-pyrrolin-2-one derivatives on cyclooxygenase 1 and 2 in murine peritoneal macrophages. Acta Pharmacol Sin 2002;23:762-8.