Binding of PLCδ1PH-GFP to PtdIns(4,5)P2 prevents inhibition of phospholipase C-mediated hydrolysis of PtdIns(4,5)P2 by neomycin1
Introduction
Phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2], a minor phospholipid component of the plasma membrane, is a key regulator of several cellular processes, and has become the focus of research on intracellular signal transduction. PtdIns(4,5)P2 is a precursor of important second messengers, such as the diffusible InsP3, which regulates Ca2+ release from intracellular Ca2+ stores, and the protein kinase C activator, diacylglycerol[1,2]. PtdIns(4,5)P2 is also phosphorylated by class I PtdIns 3-kinases to form PtdIns(3,4,5)P3, which controls membrane recruitment and the functions of several important signaling proteins[3]. PtdIns(4,5)P2 itself is a regulator of a great variety of target molecules, including ion channels[4,5] and several proteins that regulate actin polymerization and the cytoskeleton[6], providing a link between the plasma membrane and the cortical cytoskeleton[7]. PtdIns(4,5)P2 has also been implicated in several forms of membrane remodeling events, including the fusion of secretory vesicles with the plasma membrane[8], clathrin-mediated endocytosis[9], and membrane recovery by endocytosis during neurotransmitter release[10]. Such diverse functions rely upon interaction of the lipid with a large number of regulator molecules.
Pleckstrin homology (PH) domains have been described in a large number of signaling proteins, and they show remarkable specificity in recognizing various forms of inositides[11]. The PH domain of phospholipase Cδ1 (PLCδ1PH) binds with high affinity and selectivity to PtdIns(4,5)P2[12]. Recently, a fusion construct of PLCδ1PH with enhanced green fluorescent protein (GFP) (PLCδ1PH-GFP) was developed as a probe to visualize PtdIns(4,5)P2 in single cells because it binds to PtdIns(4,5)P2 within the plasma and translocates to the cytoplasm after receptor stimulation. Subsequently, when PtdIns(4,5)P2 is resynthesized, fluorescence returns to the membrane[13].
It has been demonstrated that neomycin, an aminoglycoside antibiotic with a large positive charge (about +4.5), binds with high affinity to PtdIns(4,5)P2[14]. Later studies also showed that neomycin bound to and neutralized the negative charge of PtdIns(4,5)P2[15].
Phospholipase C (PLC)-induced PtdIns(4,5)P2 hydrolysis is an important cell signaling mechanism. Many membrane receptors couple to PLC, and thus regulate PtdIns(4,5)P2 turnover and subsequent downstream cell signaling[16]. A few PLC modulators have been developed and they play an important role in understanding the cell signaling process involving PLC and PtdIns(4,5)P2. Neomycin has long been used as a blocker of PLC, although it actually binds to PtdIns(4,5)P2 and presumably prevents PtdIns(4,5)P2 from hydrolysis by PLC[17]. Previous studies have demonstrated that both PLCδ1PH and neomycin bind PtdIns(4,5)P2 through an electrostatic interaction[12,14]. This similar nature of interaction would indicate a similar consequence for PtdIns(4,5)P2 hydrolysis by PLC. However, in the present study, we demonstrate that although both PLCδ1PH and neomycin bind to PtdIns(4,5)P2, only neomycin blocks PtdIns(4,5)P2 hydrolysis by PLC activation.
Materials and methods
Reagents and plasmids Acetylcholine (ACh), bradykinin (BK), wortmannin, neomycin and Fluo 3-AM, the calcium indicators, were purchased from Sigma-Aldrich (St Louis, MO, USA). ACh, BK and neomycin were dissolved in distilled water. U73122 was purchased from Calbiochem (San Diego, CA, USA). U73122 and wortmannin were prepared as stock solutions in dimethylsulfoxide (Me2SO), with a final concentration of Me2SO of 0.1%. Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) were products of Hyclone (Logan, UT, USA). COS-7 was obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. cDNA from the type 1 muscarinic (M1) receptor (M1R) and bradykinin 2 receptor (BK2R), pEGFP-N1(GFP) and the PLCδ1PH construct with GFP (PLCδ1PH-GFP) were gifts from Prof DE LOGOTHETIS (Mount Sinai Medical School, NY, USA). Red fluorescent protein (pDsRed-Express-C1, pDsRed) was purchased from Clontech (Mountain View, CA, USA). All other chemicals were of high performance liquid chromatography or analytical grade.
Cell culture and transfection COS-7 cells were seeded in 24-well plates on 12-mm glass coverslips, and cultured in 0.3 mL of DMEM supplemented with 10% (v/v) FBS, 100 µg/mL streptomycin, and 100 U/mL of penicillin at 5% CO2 and 37 °C. When they were 60%–70% confluent, the cells were transiently transfected with DNA constructs for 8 h using calcium phosphate precipitate, with 1 µg of DNA and equal proportions for each kind of plasmid per well. Following transfection, cells were incubated in 10% FBS DMEM for 12–48 h. For fluorescence detection, cells were washed twice with a modified Krebs-Ringer buffer containing (in mmol/L): 120 NaCl, 4.7 KCl, 0.7 MgSO4, 1.2 CaCl2, 10 glucose, with 10 N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) added (pH 7.4). The coverslips were placed into a flow-through chamber and mounted on an inverted microscope.
Confocal microscopy and image analysis For confocal imaging, a Leica (Wetzlar, Germany) DM-IRBE inverted microscope with a 20×objective (numerical aperture 0.7) and fitted with a TCS-SP2 scanhead was used. Excitation of PLCδ1PH-GFP and Fluo 3-AM was achieved with a 488 nm argon ion laserline, and emissions were collected at 500–565 nm. pDsRed fluorescence was visualized with excitation at 543 nm and a 570–600 nm emission filter. For translocation studies, a series of confocal images were taken at 3–10 s intervals and stored on disk. Determination of the ratio of membrane to cytosolic fluorescence was carried out by assigning regions of interest for membrane and cytosol. TCS-SP2 confocal software (Leica) was used to analyze data off-line.
Measurement of intracellular Ca2+ ([Ca2+]i) of single cells Cells were seeded onto sterile 12-mm borosilicate coverslips in 35-mm Petri dishes, and incubated with 5 mmol/L fluo 3-AM at 37 ℃ for 45 min. After loading, cells were washed twice and maintained in modified Krebs-Ringer buffer until assay. [Ca2+]i changes were represented by relative fluorescence intensity calculated by using the equation ΔF/F0, where ΔF and F0 are the change in fluorescence intensity before and after treatment, and the initial fluorescence intensity, respectively[18,19].
Statistics Data were analyzed by using the Chi-square test. P<0.05 was considered to be a statistically significant difference. All data shown are the mean value of at least 5 experiments and are expressed as mean±SD.
Results
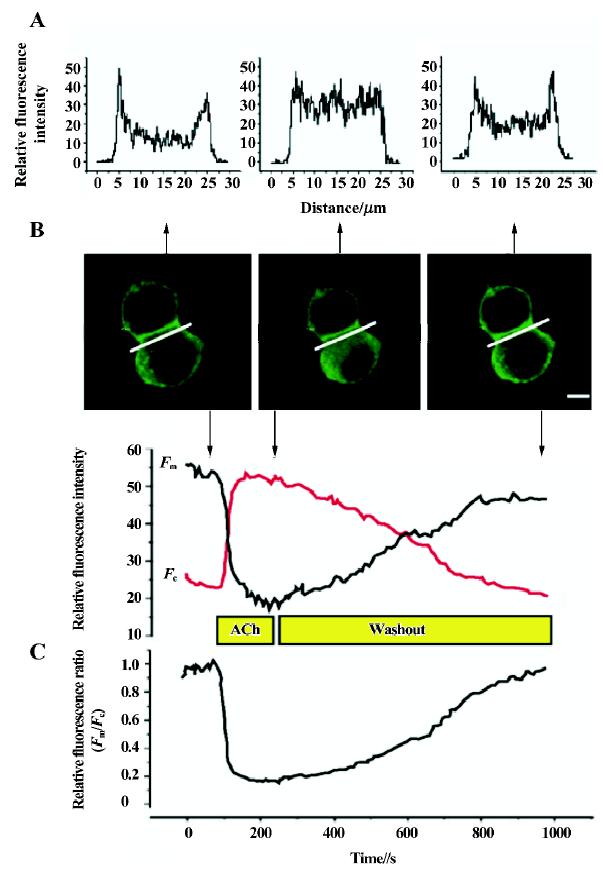
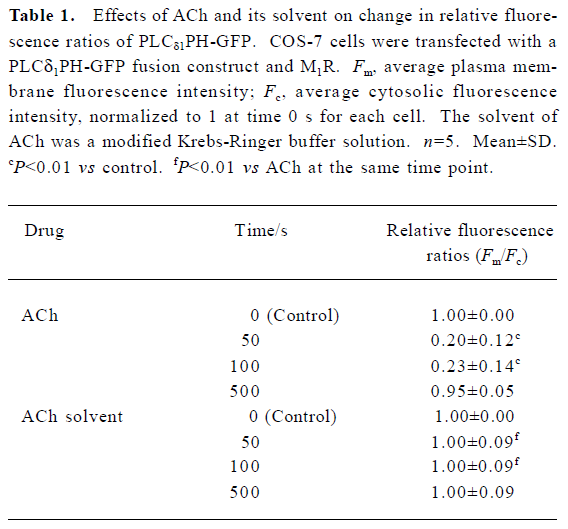
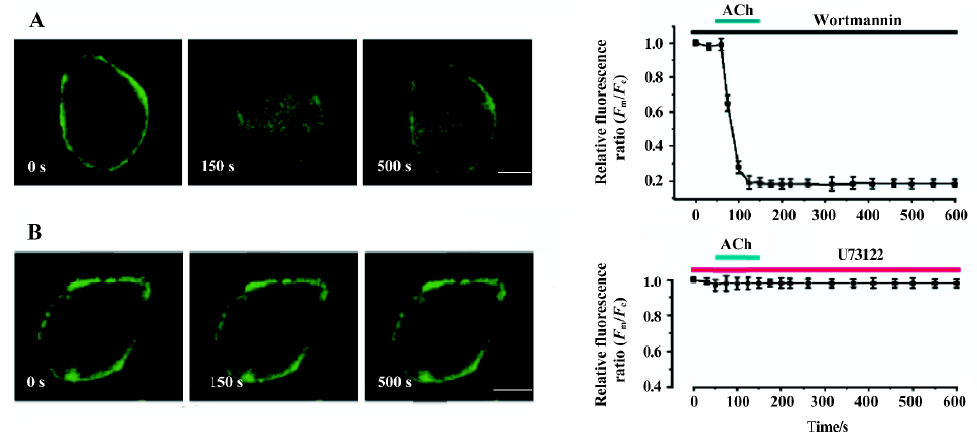
Activation of M1R and BK2R induced PtdIns(4,5)P2 hydrolysis and a reversible translocation of PLCδ1PH-GFP Both M1R, and PLCδ1PH-GFP or both BK2 and PLCδ1PH-GFP were expressed in COS-7 cells. To follow the localization of PLCδ1PH-GFP within intact cells, we used GFP as a control. In unstimulated cells, expressed GFP was found to be cytosolic and also present in the nucleus (data not shown). PLCδ1PH-GFP, on the other hand, accumulated strongly at the plasma membrane and had a low and homogenous distribution in the cytosol (Figure 1A, 1B, left panel), consistent with the hypothesis that the large pool of PtdIns(4,5)P2 exists in the plasma membrane[13]. Next we examined the effects of ACh and BK, acting through their respective G protein-linked receptors, and subsequent activation of phospholipase Cβ and hydrolysis of PtdIns(4,5)P2, on the fluorescence distribution of the GFP and PLCδ1PH-GFP. COS-7 cells were transfected with the GFP or PLC1PH-GFP together with the cDNA encoding the M1R or BK2R. After stimulation with either ACh (5 µmol/L) or BK (0.1 µmol/L), there was a decrease of PLCδ1PH-GFP fluorescence in the plasma membrane and a concomitant increase in cytosolic fluorescence (Figure 1A, 1B, Table 1). The kinetics of ACh- or BK-induced PLCδ1PH-GFP fluorescence translocation were characterized by a rapid onset, with translocation peaking at approximately 30–60 s and returning to baseline approximately 5–8 min after washout (Figure 1C). No significant change in fluorescence were seen in cells transfected with GFP only (data not shown). To exclude the effects of the laser, we used modified Krebs-Ringer buffer solution as a solvent control of ACh. As shown in Table 1, there was no change in the relative fluorescence ratios (Fm/Fc) in the solvent control group during perfusion. To examine whether ACh- or BK-induced translocation of PLCδ1PH-GFP is due to the hydrolysis of PtdIns(4,5)P2, we utilized 2 different PtdIns(4,5)P2 resynthesis and hydrolysis blockers: wortmannin and U73122. Wortmannin is known to be able to block the PtdIns 3-kinase at low concentrations and block the PtdIns 4-kinase, so therefore block the formation of PtdIns(4,5)P2 from phosphatidylinositol (PI), at high concentrations[20]. As shown in Figure 2, in COS-7 cells expressing PLCδ1PH-GFP and the M1R, after the cell was pre-incubated with wortmannin (at 10 µmol/L, a concentration known to block PtdIns 4- kinase) for 20 min, ACh induced a similar translocation of fluorescence from the plasma membrane to the cytosol, which lasted for more than 10 min after washout of ACh (Figure 2A). When the cells were pre-incubated with U73122 (10 µmol/L), a relatively specific PLC inhibitor, for 5 min, ACh failed to induce transient translocation of the fluorescence signal (Figure 2B). These data strongly suggest that PLCδ1PH-GFP translocation induced by membrane receptor activation is indeed due to PtdIns(4,5)P2 hydrolysis.
Full table
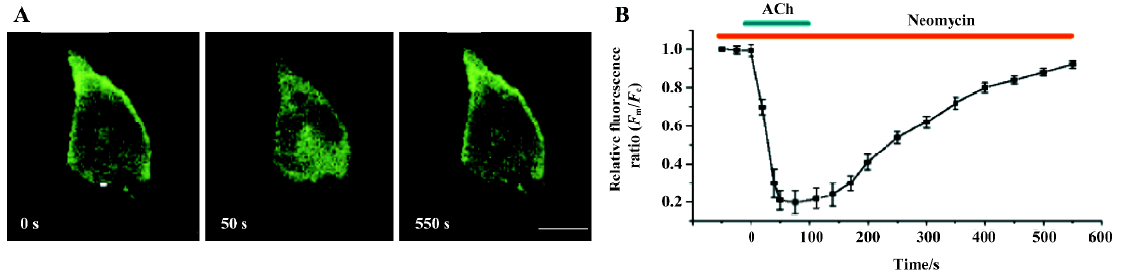
Expression of PLCδ1PH-GFP inhibited the effects of neomycin on PtdIns(4,5)P2 hydrolysis Neomycin binds PtdIns(4,5)P2 with high affinity and has often been used as an inhibitor of PLC. The blocking effect of neomycin on PLC is believed to be indirect, the result of neomycin binding to PtdIns(4,5)P2, the substrate of PLC[15]. As we shown earlier, PLCδ1PH bound to PtdIns(4,5)P2 but did not block receptor-mediated PLC activation, or PtdIns(4,5)P2 hydrolysis. Because both PLCδ1PH and neomycin bind PtdIns(4,5)P2 in a similar way (electrostatic interaction, see Introduction), we thought this difference between PLCδ1PH and neomycin was interesting, and worthy of further investigation. To determine whether the binding of PLCδ1PH-GFP to PtdIns(4,5)P2 can disrupt the effects of neomycin on PtdIns(4,5)P2 hydrolysis, COS-7 cells expressing PLCδ1PH-GFP and M1R were stimulated with ACh in the absence or presence of neomycin. Preincubation of the cells with neomycin (5 mmol/L) for 40 min failed to prevent the release of the fluorescence signal from the membrane to the cytosol upon the application of ACh (Figure 3). Thus in the presence of PLCδ1PH, neomycin could not block hydrolysis of PtdIns(4,5)P2 induced by PLC.
Effects of neomycin on PLC activation in the absence of PLCδ1PH-GFP To further confirm that binding of PLCδ1PH-GFP to PtdIns(4,5)P2 excludes the binding of neomycin to PtdIns(4,5)P2, thus blocking neomycin’s inhibitory effects on PLC, we used [Ca2+]i as an indicator to reveal the effects of neomycin on PLC in the absence of PLCδ1PH-GFP. One of the downstream products of PtdIns(4,5)P2 hydrolyzed by PLC is IP3, which acts to release intracellular Ca2+[1,2]. Thus [Ca2+]i would serve as a good indicator of PLC activation upon membrane receptor (M1R) stimulation. ACh induced a significant increase in [Ca2+]i in COS-7 cells expressing the M1R alone and pretreated with modified Krebs-Ringer buffer solution for 40 min (Figure 4A, 4B); pDsRed was co-transfected with M1R as a transfection tag (Figure 4A). However, when cells were pretreated with 5 mmol/L neomycin (40 min), no change was seen upon application of ACh (Figure 4A, 4B). Similar results were seen with BK as activator of PLC in cells expressing B2R (data not shown). Thus, in the absence of PLCδ1PH, neomycin was able to block activation of PLC.
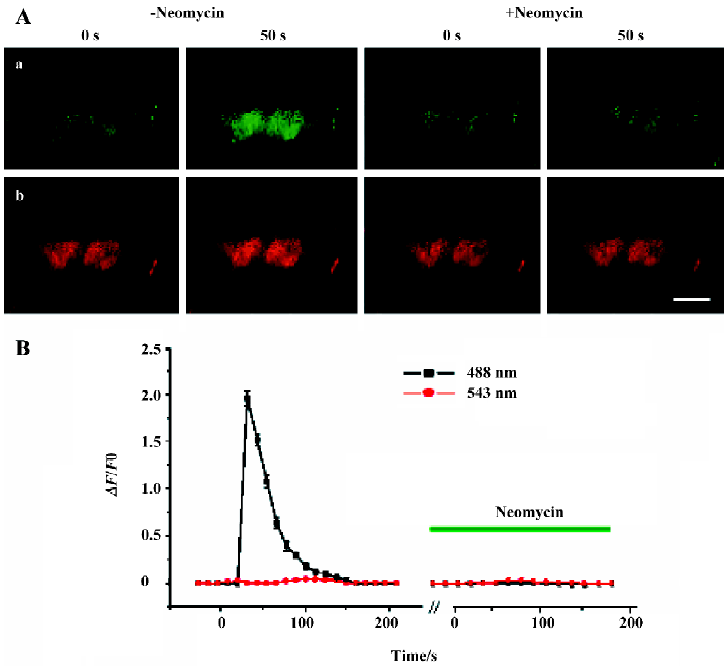
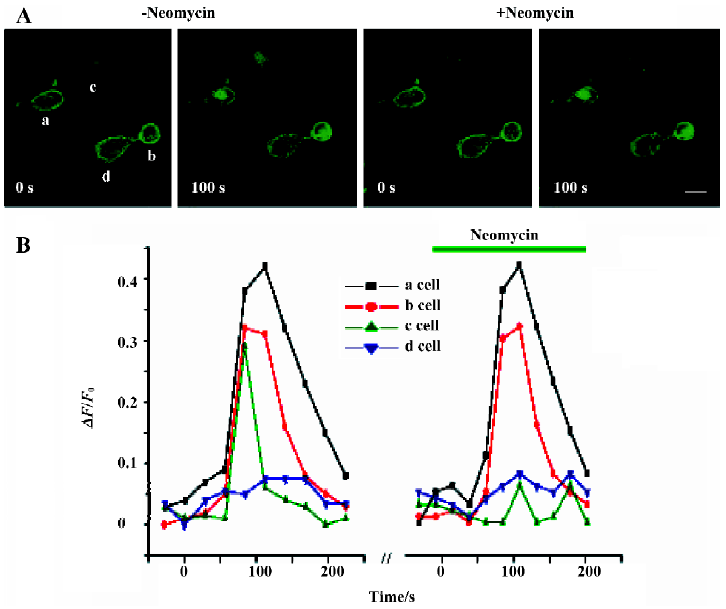
To further confirm these findings, we next examined whether neomycin could also exert its inhibitory effects on [Ca2+]i in the presence of PLCδ1PH-GFP. In this section of the study, we imaged the whole-cell fluorescence intensity changes. GFP and Fluo 3-AM were excited and imaged at the same wavelength. However, as shown in Figure 1C, the total GFP signal from one cell did not change during translocation, thus we were able to see an additional fluorescence signal from Fluo 3-AM (Ca2+) when Ca2+ was released from the store by IP3. Figure 5 shows COS-7 cells transfected with PLCδ1PH-GFP and the M1R. Three types of cells, presumably representing different transfection results, can be identified. Cells designated a and b (Figure 5A) represent those cells that had been transfected with both PLCδ1PH-GFP and the M1R, giving a clear and dominant localization of the GFP signal on the cell membrane (Figure 5A), which translocated into the cytosol upon application of ACh (Figure 5A); cell c represents cells that had only been transfected with M1R, with no visible localization of the PLCδ1PH-GFP signal on the cell membrane, and a clear rising in [Ca2+]i signal seen upon application of ACh; cell d represents cells that had been transfected with PLCδ1PH-GFP but not the M1R, so that the clear PLCδ1PH-GFP signal was not released from the membrane, and neither could an increase in [Ca2+]i signal be seen upon application of ACh (Figure 5A). When these cells were pretreated with neomycin, only the response of cell c to ACh was blocked, whereas the responses of cells a and b were unaffected. These results strongly suggest that neomycin blocks PLC activation only in the absence of PLCδ1PH-GFP.
Discussion
The main finding of the present study was that in the cells expressing PLCδ1PH-GFP, neomycin could not exhibit its inhibitory effects on PtdIns(4,5)P2 hydrolysis by PLC. There is increasing interest in understanding the actions of inositol phospholipids, especially PtdIns(4,5)P2, in living cells[21]. PtdIns(4,5)P2 participates in many cellular functions, including exocytosis, cytoskeletal function and membrane transporter and ion channel functions[4]. Many molecules have been found to be able to bind to phospholipids, and more specifically to PtdIns(4,5)P2, which forms the basis of modulation by this lipid[22]. The PH domain of PLCδ1 is one of these molecules that are believed to selectively bind to PtdIns(4,5)P2[12]. Recently, a fusion construct of PLCδ1PH with enhanced green fluorescent protein (PLCδ1PH-GFP) was developed as a probe to visualize PtdIns(4,5)P2 in single cells. This novel methodology allowed imaging and analysis of spatiotemporal changes in PtdIns(4,5)P2 in single living cells, and has been used increasingly in efforts to understand the role PtdIns(4,5)P2 plays in cell signaling, and protein function regulation[13]. The central idea behind this methodology is that the GFP signal that has been linked to PLCδ1PH will faithfully follow the dynamic changes of PtdIns(4,5)P2 during its metabolism, including during hydrolysis by PLC. However, because PLCδ1PH also binds to IP3, a downstream product of PtdIns(4,5)P2 hydrolysis, with higher affinity, some have proposed that rather than being a faithful PtdIns(4,5)P2 follower, PLCδ1PH-GFP molecules during PtdIns(4,5)P2 hydrolysis are more likely to bind to newly produced IP3[18]. But van der Wal et al showed that physiological increases in IP3 (10–100 µmol/L) on activation of PLC could not be solely responsible for the translocation of PLCδ1PH-GFP[23]. Our data presented in Figure 2 are in agreement with the results of van der Wal et al. In the cells expressing PLCδ1PH-GFP as well as the BK2 or M1 receptors, BK or ACh induced the reversible translocation of PLCδ1PH-GFP from the plasma membrane to the cytosol. Thus, although it bound to PtdIns(4,5)P2, PLCδ1PH-GFP did not interfere with cleavage of PtdIns(4,5)P2 by PLC. On the other hand, neomycin, a commonly used PLC blocker, is believed to block PLC cleavage of PtdIns(4,5)P2 by preventing PtdIns(4,5)P2 from accessing PLC[17]. It is interesting to note that whereas a smaller molecule such as neomycin would mask PtdIns(4,5)P2 from PLC cleavage, a much bigger molecule such as PLCδ1PH-GFP would allow the cleavage to happen. It is also interesting to consider that the expression of PLCδ1PH-GFP blocked the action of neomycin (Figure 3), suggesting that PLCδ1PH-GFP and neomycin bind to the same sites on PtdIns(4,5)P2. Previous studies have demonstrated that both PLCδ1PH-GFP and neomycin interact with PtdIns(4,5)P2 in an electrostatic way[12,14]. Thus the charged inositide head group of PtdIns(4,5)P2 is the binding site for both PLCδ1PH-GFP and neomycin[24], yet binding of PLCδ1PH-GFP or neomycin to PtdIns(4,5)P2 has very different consequences for PLC hydrolysis of PtdIns(4,5)P2. Although it is less likely, it needs to be noted that the GFP, rather than PLCδ1PH, may block the binding of neomycin to PtdIns(4,5)P2 through a spatial blocking effect. For many cellular proteins that have been known to interact with, and whose functions are regulated by, PtdIns(4,5)P2, the molecular basis for the interaction remains to be elucidated. Less clear is the mechanism for PtdIns(4,5)P2 hydrolysis by PLC. The present study provides interesting and stimulating information for further understanding protein-PtdIns(4,5)P2 interactions and PtdIns(4,5)P2 hydrolysis by PLC. We are currently investigating the mechanism underlying the different consequences of PtdIns(4,5)P2 binding to PLCδ1PH-GFP or neomycin with respect to its hydrolysis by PLC.
Acknowledgements
We thank Diomedes E LOGOTHETIS (Mount Sinai School of Medicine, New York University, NY, USA), for providing us with plasmids of the M1R, BK2R and PLCδ1PH-GFP, and members of the Logothetis laboratory for helpful discussions on this work.
References
- Takano M, Kuratomi S. Regulation of cardiac inwardly rectifying potassium channels by membrane lipid metabolism. Prog Biophys Mol Biol 2003;81:67-9.
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 1988;34:661-5.
- Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002;296:1655-7.
- Zhang H, He C, Yan X, Mirshaki T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol 1999;1:183-8.
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP with ion channels and transporters. Sci STKE 2001; 111: RE 19.
- Berridge PA, Xian W, Flanagan LA. Controlling cytoskeleton structure by phosphoinositide-protein interactions: phosphoino-sitide binding protein domains and effects of lipid packing. Chem Phys Lipids 1999;101:93-7.
- Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell 2000;100:221-8.
- Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, et al. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature 1995;374:173-7.
- Jost M, Simpson F, Kavran JM, Lemmon MA, Schmid SL. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated reside formation. Curr Biol 1998;8:1399-402.
- Martin TF. PtdIns(4,5)P2 regulation of surface membrane traffic. Curr Opin Cell Biol 2001;13:493-9.
- Harlan JE, Hajduk PJ, Yoon HS, Fesik SW. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature 1994;371:168-70.
- Cullen PJ, Cozier GE, Banting G, Mellor H. Modular phosphoino-sitide-binding domains: their role in signaling and membrane trafficking. Curr Biol 2001;11:R882-93.
- Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton’s tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem 1999;274:10983-9.
- Schacht J. Inhibition by neomycin of polyphosphoinositide turnover in subcellular fractions of guinea-pig cerebral cortex in vitro. J Neurochem 1976;27:1119-24.
- Gabev E, Kasianowicz J, Abbott T, McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2). Biochim Biophys Acta 1989;979:105-2.
- Lei Q, Jones MB, Talley EM, Garrison JC, Bayliss DA. Molecular mechanisms mediating inhibition of G protein-coupled inwardly-rectifying K+ channels. Mol Cell 2003;15:1-9.
- Cockcroft S, Howell TW, Gomperts B. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polypho-sphoinositide phosphodiesterase and exocytosis. J Cell Biol 1987;105:2745-50.
- Hirose K, Kadowaki S, Tanabe M, Takeshima H, Lino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science 1999;284:1527-30.
- Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P2 at fertilization of mouse eggs. Cell Sci 2002;115:2139-49.
- Nakanishi S. Catt kJ, Balla T. A wortmannin-sensitive phosphati-dylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci USA 1995;92:5317-21.
- Martin TF. PI (4,5)P (2) regulation of surface membrane traffic. Curr Opin Cell Biol 2001;13:493-9.
- Hurley JH, Meyer T. Subcellular targeting by membrane lipids. Curr Opin Cell Biol 2001;13:146-52.
- van der Wal J, Habets R, Varnai P, Balla T, Jalink K. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J Biol Chem 2001;276:15337-44.
- Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Crystal structure at 2.2Å resolution of the pleckstrin homology domain from human dynamin. Cell 1994;79:199-209.
