Enzymological characterization of FIIa, a fibrinolytic enzyme from Agkistrodon acutus venom1
Introduction
Studies on snake venoms have been proceeding for a long time. It is known that fractions of snake venom exhibit a number of biological activities, such as fibrinogenolysis and/or fibrinolysis, and anti-platelet aggregation[1]. Approximately 3 kinds of enzymes from snake venoms can degrade fibrinogen, these are thrombin-like enzyme (TLE)[2], plasminogen activator[3], and fibrinolytic enzyme. Among them, fibrinolytic enzymes can directly degrade not only fibrinogen but also fibrin in vitro and in vivo. Furthermore, they are not inhibited by proteinase inhibitors in human blood. With their potential use for treating thrombotic disease the fibrinolytic enzymes have been widely investigated. The fibrinolytic enzymes have been purified from the venoms of Agkistrodon acutus[4], A piscivorus piscivorus[5], A contortrix[6], A rhodostoma[7], Bothrops jararaca[8], Crotalus atrox[9], Trimeresurus mucrosquamatus[10] and Vipera lebetina[11]. More than 70 kinds of fibrinolytic enzymes have been isolated, and novel fibrinolytic enzymes continue to be reported.
The fibrinolytic enzyme from Taiwanese Agkistrodon acutus venom was first isolated by Ou-yang and Huang[12]. In our previous work, another fibrinolytic enzyme called FIIa was purified from Anhui Agkistrodon acutus venom. FIIa can degrade fibrin and fibrinogen in vitro, and solubilize thrombus in vivo[4,13]. However, the enzymological characteristics of FIIa have not been shown clearly. In the present investigation, we mainly investigate the influences of several protease inhibitors, chelating agents, and metal ions on the fibrinogenolytic activity of FIIa. The metal content was also determined.
Materials and methods
Snake venom Lyophilized Agkistrodon acutus venom was collected from Qimen Snake Farm (Anhui, China).
Reagents DEAE-Sephadex A-50, Sephadex G-75, ethylenediamine tetracetic acid (EDTA), ethyleneglycol tetraacetic acid (EGTA), phenylmethylsulfonylfluoride (PMSF) and soybean trypsin inhibitor (SBTi) were purchased from GE Health Care (Little Chalfont, UK). Bovine fibrinogen and plasmin were from Sigma (St Louis, MO, USA). Molecular weight protein standards were from NEB (Beverly, MA, USA). All other chemicals and solvents were of analytical grade from commercial sources.
Purification of the enzyme FIIa, a fibrinolytic enzyme from Agkistrodon acutus venom, was prepared according to the method described by Liang et al[4].
Fibrinogenolytic activity assay FIIa (1 g/L, 150 µL) was incubated with 450 µL of bovine fibrinogen (1 g/L) at 37 ℃. Aliquots were taken at 5 min, 15 min, 30 min, 45 min, 1 h, 4 h and 8 h intervals, and 600 µL of a denaturing solution (10 mol/L urea, 4% sodium dodecylsulfate and 4% β-mercaptoethanol) was added and the mixture was incubated at 100 ℃ for 4 min. Each sample (20 µL) was analyzed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) using a 4% spacer gel and a 12% separation gel[14]. Human plasmin (50 U/L) was used as positive control.
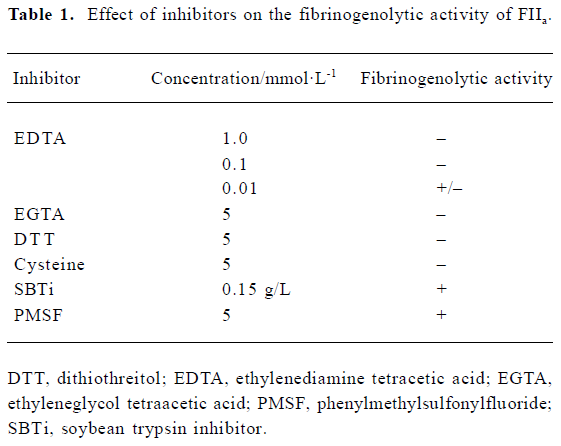
Effect of inhibitiors on fibrinogenolytic activity The effects of EDTA (5 mmol/L), EGTA (5 mmol/L), PMSF (5 mmol/L), SBTi (0.15 g/L), dithiothreitiol (DTT; 5 mmol/L) and cysteine (5 mmol/L) on fibrinogenolytic activity were examined by incubation with FIIa (1 g/L) at 37 ℃ for 1 h. After adding bovine fibrinogen (1 g/L), the mixture was incubated for a further 1 h. Each sample (20 µL) was analyzed by SDS-PAGE.
Reactivation by metal ions on fibrinogenolytic activity FIIa (1 g/L, 150 µL) was incubated with EDTA (final concentration: 5 mmol/L) at 37 ℃ for 1 h. MgCl2, CaCl2 and ZnCl2 (final concentrations: 5 mmol/L) were added to the incubation solution, and the mixture was incubated for a further 1 h. The fibrinogenolytic activity was examined by SDS-PAGE after a 1-h incubation with 450 µL of bovine fibrinogen (1 g/L). The same experiment was performed with EGTA (final concentration: 5 mmol/L) instead of EDTA.
Metal content assay Metal content was determined using an atomic absorption spectrophotometer. The absorbances of standard solutions were used to draw standard graphs. The metal content of FIIa was estimated by comparison with the standard curve[14].
Results
FIIa degraded the Aα-chain preferentially, followed by the Bβ-chain of fibrinogen, but the γ-chain was the most insusceptible to the enzyme. At a molar ration of 3:1 (fibrino-gen: FIIa), the Aα-chain was totally degraded within 5 min, with relatively lower activity for the Bβ-chain, which disappeared within 30 min. The γ-chain was only degraded following a prolonged 8-h incubation with FIIa (Figure 1A). Concomitant with the digestion of fibrinogen, major fragments of Mr approximately 45 000 and 41 000 were observed.
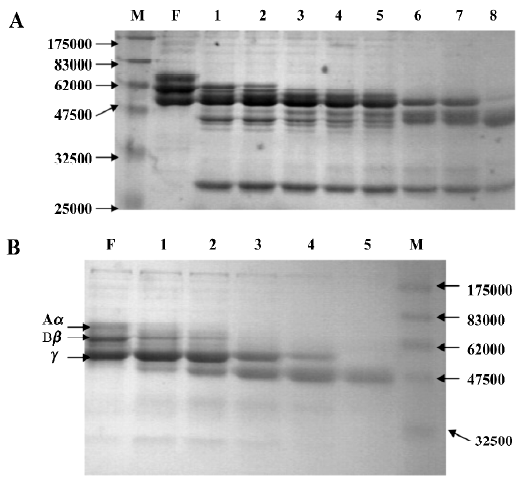
When fibrinogen was incubated with human plasmin, the Aα- and Bβ-chains disappeared within 15 min, while the γ-chain disappeared within 1 h. The major digestion fragment observed was at Mr 45 000, of which the cleavage pattern was different from that of FIIa (Figure 1B).
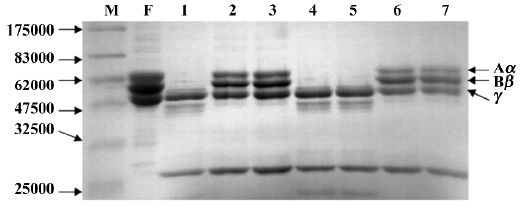
The fibrinogenolytic activity of FIIa was inhibited by EDTA, EGTA, DTT and cysteine, but not by PMSF or SBTi (Table 1). The fibrinogen was still intact after incubation with FIIa pretreated with EDTA, EGTA, DTT, and cysteine (Figure 2). However, the fibrinogen was degraded after incubation with FIIa pretreated with PMSF and SBTi (Figure 2). Zn2+, Ca2+, and Mg2+, at concentrations of 5 mmol/L, could restore the fibrinogenolytic activity of EDTA-treated FIIa. Only Ca2+ could restore the fibrinogenolytic activity of EGTA-treated FIIa. Both 1 mmol/L and 5 mmol/L Ca2+ were effective (Figure 3).
Full table

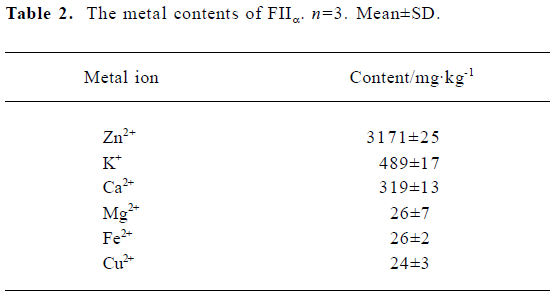
Zn2+, K+ and Ca2+ were found in significant quantities, at 3171±25 mg/kg, 489±17 mg/kg and 319±13 mg/kg, respec-tively. The concentrations of Mg2+, Fe2+ and Cu2+ were only at trace amounts (Table 2). For each mole of FIIa, there was approximately 1 mole of Zn2+, 0.3 mole of K+ and 0.2 mole of Ca2+.
Full table
Discussion
FIIa is a α,β-fibrinogenase because it degraded both the Aα-chain and the Bβ-chain of fibrinogen[15]. The Aα-chain of fibrinogen was very susceptible to FIIa, and it was completely degraded within 5 min. Cleavage of the γ-chain of fibrinogen was observed only with a prolonged incubation time. Thus far there have been few reports of fibrin(ogen)olytic snake venom enzymes that cleave of the γ-chain. No enzyme reported has shown cleavage specificity directed solely at the γ-chain[16]. Because the γ-chain of fibrinogen was stable when was incubated with snake venom fibrin(ogen)olytic enzyme, we postulated that the degradation might occur at either an increased incubation time or at an increased concentration. In our previous study, the γ-chain was unaffected after a 2-h incubation. However, in the present study FIIa appeared to degrade the γ-chain after prolonged (8 h) incubation. The same phenomenon was noticed for cerastase F-4 (from Cerastes cerastes venom) and a fibrin(ogen)olytic enzyme from V lebetina venom, and they appeared to degrade the γ-chain following 48-h and 24-h incubations, respectively[17,18]. Plasmin also cleavages the Aα-, Bβ-, and γ-chains of fibrinogen, but the patterns are different from those observed when cleaved by FIIa. It is interesting that various fibrin(ogen)olytic enzymes seem to produce different degradation patterns for fibrinogen. For example, FIIa mainly yields fragments of 45 kDa and 41 kDa, while basilase produces fragments of 45 kDa, 36 kDa and 10 kDa, and atroxase gives fragments of 45 kDa and 38 kDa[19]. The studies on some fibrin(ogen)olytic enzymes reveal that their cleavage preference is commonly directed to the amino-terminal side of hydrophobic amino acid residues. They display distinct and unique cleavage characteristics with fibrinogen.
The fibrin(ogen)olytic enzymes from snake venoms can be classified as metalloproteinases or serine proteinases. Chelating agents (EDTA, EGTA) completely inhibited FIIa, while serine protease inhibitors (PMSF, SBTi) were ineffective, indicating that it belongs to the metalloproteinase group. This was supported by data from atomic absorption spectroscopy. For each mole of FIIa there was approximately 1 mole of Zn2+, 0.3 mole of K+ and 0.2 mole of Ca2+. Like many of the venom fibrinolytic enzymes, FIIa is a zinc metalloproteinase. Besides Zn2+, Ca2+ is another metal ion often found in venom with fibrinolytic enzymes. Metal analysis has indicated that the calcium content of atroxase (from western diamondback rattlesnake venom)[9] and lebetase (from V. lebetina snake venom)[20] is 0.3 mol/mol and 1 mol/mol, respectively. In adamalysin from C. adamanteus[21] and atrolysin c(d) from C. atrox[22] it was found that except Zinc-binding site, a calcium ion is bound near the carboxy-terminus of the enzyme. Thus far, only atroxase was reported to contain 1 mol/mol of K+, while FIIa contains 0.3 mol/mol of K+. The functions of calcium and potassium have not been elucidated, but they may play a role in retaining the stability of the protein.
Zn2+, Ca2+ and Mg2+ were effective in restoring the activity of EDTA-treated FIIa, while only Ca2+ could restore the activity of EGTA-treated FIIa. The mechanism for this is not clear. It is reported that snake venom metalloproteinases have Zn2+-dependent activities, but some are more active in the presence of Ca2+[23,24]. This seems probably responsible in part for this phenomenon. The effect of Mg2+ on the activity of FIIa needs to be elucidated. FIIa is inhibited by DTT and cysteine, suggesting that disulfide bonds are necessary for holding the structure.
In conclusion, like many venom fibrin(ogen)olytic enzymes, FIIa is a metalloproteinase. Both Zn2+ and Ca2+ play important roles in the fibrinogenolytic activity of FIIa.
References
- Tseng YL, Lee CJ, Huang TF. Effects of a snake venom metallop- roteinase, triflamp, on platelet aggregation, platelet-neutrophil and neutrophil-neutrophil interactions: involvement of platelet GPIbα and neutrophil PSGL-1. Thromb Haemost 2004;91:315-24.
- Tatematsu R, Komori Y, Nikai T. A new thrombin-like enzyme, flavoviridiobin from the venom of Trimeresurus flavoviridis (habu). J Nat Toxins 2000;9:327-39.
- Zhang Y, Wisner A, Xiong Y, Bon C. A novel plasminogen activator from snake venom. Purification, characterization, and molecular cloning. J Biol Chem 1995; 270: 10 246–55.
- Liang XX, Chen JS, Zhou YN, Qiu PX, Yan GM. Purification and biochemical characterization of FIIa, a fibrinolytic enzyme from Agkistrodon acutus venom. Toxicon 2001;39:1133-9.
- Hahn BS, Chang IM, Kim YS. Purification and characterization of piscivorase I and II, the fibrinolytic enzymes from eastern cottonmouth moccasin venom (Agkistrodon piscivorus). Toxicon 1995;33:929-41.
- Trikha M, Schmitmeier S, Markland FS. Purification and characterization of fibrolase isoforms from venom of individual southern copperhead (Agkistrodon contortrix) snakes. Toxicon 1994;32:1521-31.
- Ouyang C, Hwang LJ, Huang TF. Alpha-fibrinogenase from Agkistrodon rhodostoma (Malayan pit viper) snake venom. Toxicon 1983;21:25-33.
- Maruyama M, Sugiki M, Yoshida E, Shimaya K, Mihara H. Broad substrate specificity of snake venom fibrinolytic enzymes: possible role in haemorrhage. Toxicon 1992;30:1387-97.
- Willis TW, Tu AT. Purification and biochemical characterization of atroxase, a nonhemorrhagic fibrinolytic protease from western diamondback rattlesnake venom. Biochemistry 1988;27:4769-77.
- Hung CC, Huang KF, Chiou SH. Characterization of one novel venom protease with beta-fibrinogenase activity from the Taiwan habu (Trimeresurus mucrosquamatus): purification and cDNA sequence analysis. Biochem Biophys Res Commun 1994;205:1707-15.
- Siigur E, Siigur J. Purification and characterization of lebetase, a fibrinolytic enzyme from Vipera lebetina (snake) venom. Biochim Biophys Acta 1991;1074:223-9.
- Ou-yang C, Huang TF. Purification and characterization of the fibrinolytic principle of Agkistrodon acutus venom. Biochim Biophys Acta 1976;439:146-53.
- Chen JS, Liang XX, Qiu PX, Yan GM. Thrombolysis effect with FIIa from Agkistrodon acutus venom in different thrombosis model. Acta Pharmacol Sin 2001;22:420-2.
- Gasmi A, Chabchoub A, Guermazi S, Karoui H, Elayeb M, Dellagi K. Further characterization and thrombolytic activity in a rat model of fibrinogenase from Vipera Lebetina venom. Thromb Res 1997;86:233-42.
- Pinto AF, Dobrovolski R, Veiga AB, Guimaraes JA. Lonofibrase, a novel α-fibrinogenase from Lonomia obliqua caterpillars. Thromb Res 2004;113:147-54.
- Swenson S, Markland FS Jr. Snake venom fibrin(ogen)olytic enzymes. Toxicon 2005;45:1021-39.
- Daoud E, Tu AT, el-Asmar MF. Mechanism of the anticoagulant, Cerastase F-4, isolated from Cerastes cerastes (Egyptian sand viper) venom. Thromb Res 1986;41:791-9.
- Gasmi A, Karoui M, Benlasfar Z, Karoui H, el-Ayeb M, Dellagi K. Purification and characterization of a fibrinogenase from Vipera lebetina (desert adder) venom. Toxicon 1991;29:827-36.
- Gatta D, Dong A, Witt J, Tu AT. Biochemical characterization of basilase, a fibrinolytic enzyme from Crotalus basiliscus. Arch Biochem Biophys 1995;317:365-73.
- Siigur J, Samel M, Tonismagi K, Subbi J, Siigur E, Tu AT. Biochemical characterization of lebetase, a direct-acting fibrinolytic enzyme from Vipera lebetina snake venom. Thromb Res 1998;90:39-49.
- Gomis-Ruth FX, Kress LF, Bode W. First structure of a snake venom metalloproteinase: a prototype for matrix metalloproteinase/collagenase. EMBO J 1993;12:4151-7.
- Zhang D, Botos I, Gomis-Ruth FX, Doll R, Blood C, Njoroge FG, et al. Structural interaction of natural and synthetic inhibitors with the venom metalloproteinase, atrolysin c (from d). Proc Natl Acad Sci USA 1994;91:8447-51.
- Assakura MT, Reichl AP, Asperti MC, Mandelbaum FR. Isolation of the major proteolytic enzyme from the venom of the snake Bothrops moojeni (caissaca). Toxicon 1985;23:691-706.
- De-Camargo-Goncalves LR, Chudzinski-Tavassi AM. High molecular mass kininogen inhibits metalloproteinases of Bothrops jararaca snake venom. Biochem Biophys Res Commun 2004;318:53-9.
