Neuroprotective effects of scutellarin on rat neuronal damage induced by cerebral ischemia/reperfusion
Introduction
Cerebral ischemia is accompanied by a marked inflammatory process, which is initiated by higher levels of expression of cytokines, adhesion molecules, and other inflammatory mediators, including nitric oxide[1,2]. Recent studies have demonstrated that nitric oxide (NO) and pro-inflammatory cytokines released by microglial cells, which act as resident macrophage-like cells in the brain, are partly responsible for neuronal cell death. NO levels are associated directly with the development of brain injury in strokes and other neuropathological disorders in humans[3,4].
Following acute ischemic or hypoxic injury to the brain, over-entry of Ca2+ into cells causes the activation of nitric oxide synthase (NOS), which catalyzes an enzymatic reaction, leading to the synthesis of nitric oxide[5–7]. Three kinds of distinct NOS isoforms have been identified, including neuronal nNOS, endothelial eNOS, and an inducible isoform, iNOS, originally isolated from macrophages. nNOS and eNOS are constitutively expressed and calcium-dependent, whereas iNOS is expressed in response to various inflammatory stimuli, and its activity is independent of intracellular calcium concentrations. NO can be neuroprotective or neurotoxic during cerebral ischemia, depending on the NOS isoform involved. eNOS produces NO with beneficial effects (vasodilation, inhibition of platelet aggregation and polymorphonuclear neutrophil adhesion)[7–9], whereas NO overproduction by nNOS or iNOS during ischemia is cytotoxic. Based on these findings, it thought that the NO-synthases could be attractive targets for treating cerebral ischemia-induced neuronal damage[10,11].
Scutellarin, a flavonoid, is the major active ingredient extracted from Erigeron breviscapus Hand Mazz, a plant used in Chinese herbal medicine, which is a Ca2+-channel-blocking agent used for the clinical treatment of cerebrovascular disorders. Studies have demonstrated the protective effects of scutellarin on brain injury induced by cerebral ischemia/reperfusion (I/R) through interaction with a wide variety of targets because of its anti-oxidative and anti-inflammatory actions, and its ability to attenuate neuronal damage[12,13]. It is presumed that these effects might be related to the effects of scutellarin on the NO synthases. Therefore, the objective of the present study was to elucidate the effects of scutellarin on the expression of the NOS isoforms (iNOS, eNOS, nNOS) in a model of cerebral I/R in rats. Moreover, the key angiogenic molecules, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), were also studied.
Materials and methods
Chemicals and drugs Scutellarin was supplied by Yuxi Pharmaceuticals (Kunming, China). The purity of this compound was more than 96% and it was dissolved in saline before use. 2,3,5-Triphenyltetrazolium chloride (TTC, N
Animal treatment and administration Male Sprague-Dawley rats (Grade II, Certificate N
Cerebral I/R procedure Rats were anesthetized with chloral hydrate (300 mg/kg, ip). Brain I/R injury was induced by a middle cerebral artery occlusion (MCAO) as described previously[14]. Briefly, the right common carotid artery, external carotid artery (ECA) and internal carotid artery (ICA) were isolated via a ventral midline incision. A 50 mm length of monofilament nylon suture (ϕ 0.22–0.24 mm), with its tip rounded by heating near a flame, was introduced into the ECA lumen and advanced into the ICA for a distance of 18−20 mm in order to block the origin of the MCA. The body temperature of the rats was maintained at 36.5–37.5 ºC during the surgical procedure with an infra red heat lamp. Sham-operated animals were not exposed to I/R. After 2 h of ischemia, the nylon suture was withdrawn to establish reperfusion. After arousal from anesthesia, the rats were returned to the cages.
Behavioral testing and measurement of infarct area After 24 h reperfusion, the neurological deficit score of each rat was obtained according to Longa’s method[14] by a single experimenter, who was blinded to the experimental treatment groups. The neurological findings were scored on a 5-point scale: no neurological deficit=0, failure to extend right paw fully =1, circling to right=2, falling to right=3, did not walk spontaneously and had depressed levels of conscious-ness=4. Then the rats were anesthetized with 10% chloral hydrate (350 mg/kg) ip and subsequently decapitated. The brains were removed for measurement of infarct volume by using the TTC staining method. Five thin sections were selected from the thick slices at 2 mm intervals (from the anterior 5 mm to the anterior 13 mm) to determine the infarct areas. The slices were immersed in 2% triphenyltetrazolium chloride in saline and incubated at 37 ºC for 20 min, and then fixed with 10% formaldehyde (Sigma) neutral buffer solution (pH 7.4). At that time, the infarct tissue was unstained, whereas the normal part was stained red. Using a computerized image analysis system (NIH Image, Version 1.61), the infarct areas on each slice were summed and multiplied by slice thickness to give the infarct volume, and then expressed as the percentage of infarction per ipsilateral hemisphere.
Evaluation of permeability of blood-brain barrier The integrity of the blood-brain barrier (BBB) was investigated using Evans blue (EB) dye extravasation, according to the method of Matsuo et al[15]. Briefly, after 6-h reperfusion, the rats were treated with EB dye (2% in saline, 3 mg/kg iv). After 45 min, the rats were anesthetized with 10% chloral hydrate (350 mg/kg ip) and then the rats’ chests were subsequently opened. Physiological saline was perfused through the left ventricle until a colorless perfusion fluid was obtained from the right atrium. The cranial vault was opened, and the brain was removed, weighed (wet tissue) and placed in a 50% trichloroacetic acid solution. After homogenization and centrifugation, the supernatant (extracted dye) was diluted with ethanol (1:3) and its fluorescence was determined (excitation at 620 nm and emission at 680 nm) with a luminescence spectrometer (Hitachi, Tokyo, Japan). Calculations of the amount of EB dye in the tissue were based on a linear standard curve and were expressed per gram of tissue.
Determination of total NOx content in brain tissue At the end of 2 h ischemia and 24 h reperfusion, the rats were decapitated and the ischemic hemispheres were removed for assay of the NO level in ischemic brain tissue. The levels of metabolic products (NO2 and NO3) in vivo were determined by using a chemiluminescent NO detector (Siever 280i) as described previously[16].
Western blot analysis Western blot analysis was performed after 24 h reperfusion. The rats’ brains were removed and the ischemic hemispheres were used for assay of the protein expression of iNOS, eNOS, nNOS, VEGF and bFGF. The hippocampus and the cortex were quickly isolated and rinsed in sterilized water on ice, and then stored at -80 °C until use. Protein determination was performed according to the Lowry method. The obtained protein samples were subjected to 15% sodium dodecylsulfate-polyacrylamide gel electrophoresis, using 7.5%–15% polyacrylamide gel, and electrotransferred to polyvinyli-dene difluoride filter (PVDF) membranes. To reduce non-specific binding, the PVDF was blocked for 2 h at room temperature with 5% non-fat milk in phosphate-buffered saline (PBS). Then membranes were incubated overnight at 4 °C with the primary antibodies for iNOS (anti-rabbit iNOS mouse monoclonal antibody, 1:200 dilution, Santa Cruz), eNOS (anti-human eNOS rabbit polyclonal antibody, 1:200 dilution, Affinity Bioreagents), nNOS (anti-human nNOS rabbit polyclonal antibody, 1:200 dilution, Sanying Biotechnology), VEGF (antihuman VEGF rabbit polyclonal antibody, 1:200 dilution, Santa Cruz) or bFGF (anti-human bFGF rabbit polyclonal antibody, 1:500 dilution, Santa Cruz), respectively. After incubation with the antibodies, the membranes were washed with PBS-Tween-20 (PBS-T: 10 mmol/L phosphate buffer, pH 7.4, 150 mmol/L NaCl, 0.05% Tween 20) for 30 min and incubated in the relevant horseradish peroxidase-conjugated secondary antibody (1:600 dilution) for 30 min. The membranes were washed again with PBS-T and immunoreactive protein bands were visualized using the enhanced chemiluminescence detection system.
Statistical analysis Data are expressed as mean±SD and analyzed by using Microsoft Excel 2002. Statistical analyses were performed by using Student’s t-test. P<0.05 was considered significant.
Results
Effects of scutellarin on the infarct area, neurological score and the permeability of the blood-brain barrier Scutellarin (50 or 75 mg/kg) significantly reduced the infarct area and ameliorated the neurological deficit (P<0.05 or P<0.01 vs vehicle-operated group) (Table 1). The EB content of brain tissue after I/R for sham-operated, vehicle-operated and scutellarin-operated groups (25, 50, or 75 mg/kg) was 3.83±1.03, 8.45±1.67, 7.45±1.77, 5.02±1.12, and 4.45±1.05, respectively (Figure 1). There was a significant increase in the permeability of the BBB in rats in the vehicle-treated group compared with the sham-treated group (P<0.01). Scutellarin (50 or 75 mg/kg) obviously inhibited the increased EB content induced by cerebral I/R, and there was no obvious difference between the 2 doses.
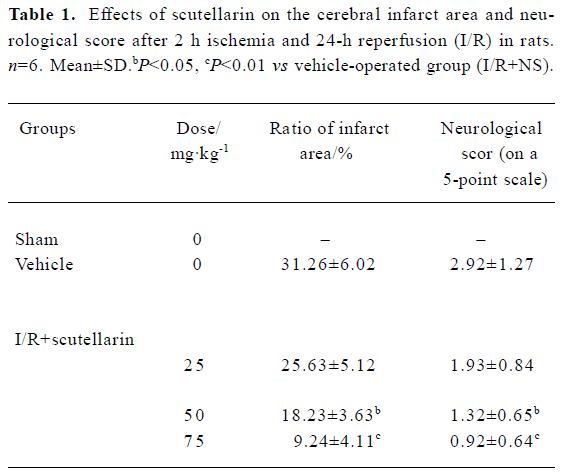
Full table
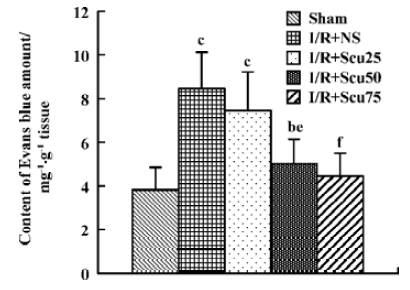
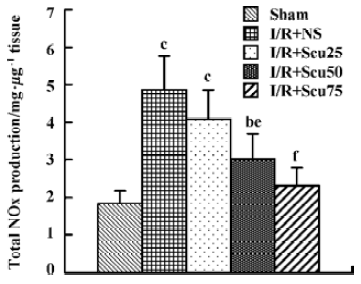
Effects of scutellarin on total NOx production After cerebral I/R, total NOx production, as determined by NOx content in the ischemic brain hemispheres, was markedly increased in the vehicle rats (4.87±0.90) compared with the sham-treated rats (1.83±0.34) (P<0.01, Figure 2). Total NOx production in rats pretreated with scutellarin at concentrations of 50 or 75 mg/kg (3.01±0.68, 2.31±0.48), were significantly reduced compared with the vehicle-operated group (P<0.05 or P<0.01, respectively) (Figure 2).
After 24 h reperfusion, the expression levels of iNOS, eNOS and nNOS were detected in the hippocampus and in the cortex, with molecular masses of 130, 140, 160 kDa, respectively. In the vehicle-treated group, the expression levels of the 3 NO synthases in the hippocampus and in the cortex markedly increased after cerebral I/R (P<0.01 or P<0.05, Figure 3A, 3B, lane 2). Densitometric analysis showed that the protein levels of eNOS and iNOS in the scutellarin-treated (50 or 75 mg/kg) rats were 368.0±70.3% and 278.0%±56.6% in the hippocampus (Figure 3A, lanes 3, 4), and 198.1%±19.2% and 148.3%±17.6% in the cortex (Figure 3B, lane 3, 4) for iNOS; and 469.0%±40.5% and 523.0%±67.3% in the hippocampus (Figure 3A, lanes 3, 4), and 188.3%±31.2% and 234.2%±37.8% in the cortex (Figure 3B, lanes 3, 4) for eNOS, respectively. The data indicate that scutellarin downregulated the expression of iNOS and simultaneously upregulated that of eNOS as compared with the vehicle-operated group (P<0.05 or P<0.01, respectively), whereas there was no difference in nNOS expression in the hippocampus or the cortex between the vehicle-treated and scutellarin-treated rats.
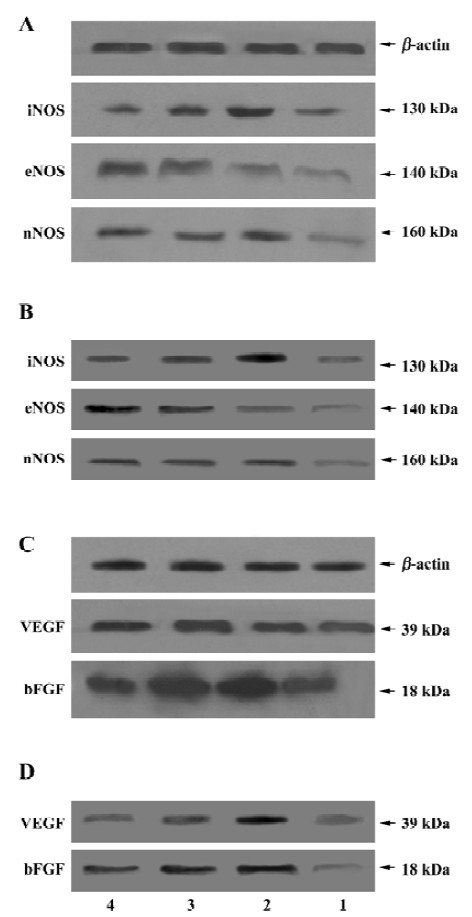
Immunoblot analysis showed single bands with molecular masses of approximately 39 and 18 kDa, which correspond to VEGF and bFGF in the hippocampus and in the cortex, respectively. The bands obtained from the vehicle-operated I/R rats (VEGF: 278.2%±43.4% in the hippocampus and 256.7%±36.8% in the cortex; bFGF: 432.2%±50.4% in the hippocampus and 289.4%±49.7% in the cortex) (Figure 3C, 3D, lane 2) were stronger than those from the sham group rats (VEGF: 111.9%±24.2% in the hippocampus and 123.9%±16.1% in the cortex; bFGF: 98.8%±15.4% in the hippocampus and 98.8%±10.9% in the cortex) (Figure 3C, 3D, lane 1, P<0.01). Scutellarin at doses of 50 or 75 mg/kg (Figure 3C, 3D, lanes 3, 4) significantly decreased the expression of VEGF and bFGF in the hippocampus and in the cortex, as compared with the vehicle-operated group (P<0.05 or P<0.01). When rats were pretreated with scutellarin at a concentration of 50 or 100 mg/kg, the VEGF protein levels in the hippocampus were 189.8%±35.4% and 123.5%±30.1%, whereas in the cortex they were 178.2%±22.1% and 145.7%±11.9%, respectively; bFGF protein levels in the hippocampus were 212.9%±33.2% and 134.5%±19.1%, while in the cortex they were 212.9%±30.4% and 145.6%±17.6%, respectively.
Discussion
In the present study, we demonstrated that scutellarin (at doses of 50 or 75 mg/kg) significantly reduced infarct volume, ameliorated the neurological deficit and reduced the permeability of the BBB after cerebral I/R. Therefore, the conclusions obtained from the above observations were that scutellarin has protective effects for the neuronal damage induced by cerebral I/R in rats.
Evidence has accumulated that NO produced both before and after cerebral ischemia may be an important factor in the pathogenesis of neuronal ischemic injury. NO is a signaling molecule that regulates many biological processes in the brain. The present paper also investigated the effects of scutellarin on the total NOx content in rat brain tissues after cerebral I/R. Our results showed that total NOx production, as determined by NOx content, in the ischemic brain hemispheres was markedly increased in cerebral I/R rats, which indicates that NO regulates the severity of cerebral ischemic injury. However, NOx content markedly decreased in brain tissues after treatment with scutellarin at doses of 50 or 75 mg/kg.
Numerous studies have been conducted regarding the differential roles of NOS isoforms and their temporal NO production in the pathogenesis of ischemic brain injury[17–19]. eNOS-derived NO is thought to be beneficial for promoting collateral circulation and microvascular flow, whereas nNOS- and iNOS-derived NO is detrimental in the ischemic brain. NO is a nontoxic agent and acts as a second messenger in normal brain; however, in the presence of O2–, NO reacts with O2– to form ONOO– or nitrogen dioxide (NO2–), causing injury to the mitochondrial electron transport system, resulting in neuronal damage. In addition, excess NO stimulates ADP ribosyltransferase and binds closely to iron-sulfur centers of enzymes, including enzymes involved in the mitochondrial electron transport chain and the tricarboxylic acid cycle (TCA), and DNA[20–22]. In view of the detrimental and beneficial roles of NOS isoforms in ischemic brain injury, further investigations into the effect of scutellarin on the expression of NOS isoforms (iNOS, eNOS, nNOS) both in the hippocampus and in the cortex after cerebral I/R were also performed in the present study. We found that expression of the NO-synthases in the hippocampus and cortex all markedly increased after cerebral I/R in the vehicle-operated group, a similar finding to those of previously studies [22–26]. Scutellarin at doses of 50 and 75 mg/kg downregulated iNOS expression and upregulated eNOS expression, which partly account for its protective effect on brain damage induced by cerebral I/R.
Increasing evidence has shown that some angiogenic molecules, including VEGF and bFGF, increase in concentration after cerebral I/R. VEGF is an angiogenesis and vascular permeability factor that undergoes transcriptional and post-transcriptional induction by hypoxia; it couples hypoxia to angiogenesis in diverse tissues, including the brain[23–26]. VEGF may also play an important role in the vascular response to cerebral ischemia, because ischemia stimulates VEGF expression in the brain, which promotes the formation of new cerebral blood vessels[27,28]. It is thought that eNOS is involved in mediating the angiogenic molecules (VEGF and bFGF). Both factors induced eNOS expression, so eNOS may be a downstream messenger in their angiogenic action. In the present study we also investigated the expression of the angiogenic molecules VEGF and bFGF in the hippocampus and in the cortex. In agreement with the results of previous reports[29,30], our data showed that the expression of VEGF and bFGF in the hippocampus and cortex were upregulated in the vehicle-operated group after cerebral I/R. Scutellarin downregulated the expression of VEGF and bFGF. Further study is needed to shed light on the mechanisms involved in the effect of scutellarin on the expression of eNOS (upregula-tion) and VEGF (downregulation) in I/R brain tissue.
In conclusion, scutellarin alleviated hippocampal neuronal dysfunction after cerebral I/R. This alleviation was accompanied by the effects of the molecular features of NOS isoforms and angiogenic molecules. These findings suggest that scutellarin exerts neuroprotective effects on brain injury induced by cerebral I/R, which allows a better understanding regarding the potential clinical therapeutic use of scutellarin.
References
- Iadecola C, Alexander M. Cerebral ischemia and inflammation. Neurology 2001;14:89-94.
- Osuka K, Watanabe Y, Usuda N, Nakazawa A, Tokuda M, Yoshida J. Modification of endothelial NO synthase through protein phosphorylation after forebrain cerebral I/R. Stroke 2004;35:2582-6.
- Naka M, Nanbu T, Kobayashi K, Kamanaka Y, Komeno M, Yanase R, et al. A potent inhibitor of inducible nitric oxide synthase, ONO-1714, a cyclic amidine derivative. Biochem Biophys Res Commun 2000;270:663-7.
- Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke 2002;33:2704-10.
- Yan XB, Meng FJ, Song B, Zhang GY. Brain ischemia induces serine phosphorylation of neuronal nitric oxide synthase by Ca (2+)/calmodulin-dependent protein kinase II in rat hippocampus. Acta Pharmacol Sin 2004;25:617-22.
- Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at Ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett 2000;477:258-62.
- Zollner S, Aberle S, Harvey SE, Polokoff MA, Rubanyi GM. Changes of endothelial nitric oxide synthase level and activity during endothelial cell proliferation. Endothelium 2000;7:169-84.
- Li L, Shen YM, Yang XS, Wu WL, Wang BG, Chen ZH, et al. Effects of spiramine T on antioxidant enzymatic activities and nitric oxide production in cerebral ischemia-reperfusion gerbils. Brain Research 2002;944:205-9.
- Sugimoto K, Iadecola C. Effects of aminoguanidine on cerebral ischemia in mice: comparison between mice with and without inducible nitric oxide synthase gene. Neurosci Lett 2002;331:25-8.
- Gajkowska B, Viron A, Cholewinski M. Immunocytochemical localization of endothelial nitric oxide synthase (e-NOS) and inducible nitric oxide synthase (i-NOS) in rat neurohypophysis after transient cerebral ischemia. Folia Neuropathol 1999;37:10-9.
- Santizo R, Baughman VL, Peligrino DA. Relative contributions from neuronal and endothelial nitric oxide synthases to regional cerebral blood flow changes during forebrain ischemia in rats. Neuroreport 2000;11:1549-53.
- Liu H, Yang XL, Wang Y, Tang XQ, Jiang DY, Xu HB. Protective effects of scutellarin on superoxide-induced oxidative stress in rat cortical synaptosomes. Acta Pharmacol Sin 2003;24:1113-7.
- Yang XF, He W, Lu WH, Zeng FD. Effects of scutellarin on liver function after brain I/R in rats. Acta Pharmacol Sin 2003;24:1118-24.
- Zea Longa E, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniotomy in rats. Stroke 1989;20:84-91.
- Matsuo Y. Mihara Si, Ninomiya M, Fujimoto M. Protective effect of endothelial type A receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke 2001;32:2143-8.
- Gao F, Gao E, Yue TL. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of P13-kinase, Akt. and endothelial nitric oxide synthase phosphorylation. Circlulation 2002;105:1497-502.
- Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovasculariza-tion after cerebral ischemia. Am J Pathol 2000;15:965-76.
- Navarro-Antolin J, Rey-Campos J, Lamas S. Transcriptional induction of endothelial nitric oxide gene by cyclosporine A. A role for activator protein-1. J Biol Chem 2000;275:3075-80.
- Shen BQ, Lee DY, Ziocheck TF. Vascular endothelial growth factor governs endothelial nitric oxide synthase via a KDR/FlK-1 receptor and a protein C kinase signaling pathway. J Biol Chem 1999;274:33057-63.
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002;8:963-70.
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Yamamoto S, Hatano O. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 2003;24:171-89.
- Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002;110:429-41.
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, et al. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol 2004;55:381-9.
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab 1996;16:9817.
- Nagayama M, Aber T, Nagayama T, Ross ME, Iadecola C. Age-dependent increase in ischemic brain injury in wild-type mice and in mice lacking the inducible nitric oxide synthase gene. J Cereb Blood Flow Metab 1999;19:661-6.
- Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci 2003;23:223-9.
- Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res 2000;119:139-53.
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab 2002;22:379-92.
- Krum JM, Mani N, Rosenstein JM. Angiogenic and astroglial responses to vascular endothelial growth factor administration in adult rat brain. Neuroscience 2002;110:589-604.
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000;106:829-38.
