Recombinant human B7-H4 expressed in Escherichia coli inhibits T lymphocyte proliferation and IL-2 secretion in vitro1
Introduction
A popular model to explain the requirement of co-signals in T-cell activation is the two-signal hypothesis[1]. In this model, signal one is derived from the T Cell Receptor after triggering by antigenic peptide presented by major histocompatibility complex (MHC) molecules and signal two is delivered through costimulation. Integration of the two signals induces the optimal activation of T cells. Lack of a co-stimulatory signal leads to decreased responses and, in some cases, even induces tolerance or energy (an unresponsive status of T cells to antigens). However, this two-signal model (or ‘missing signal model’) has been recently challenged by the discovery of new co-inhibitory pathways and their crucial roles in the control of T-cell activation. The co-inhibitors described so far function in at least three distinct co-signalling pathways: the CD80/CD86-CTLA4 axis, the B7-H1/B7-DC-PD1 axis, and B7-H4 and BTLA[2].
Chen et al identified B7-H4 (B7S1, B7X), which is a B7-family member with co-inhibitory function[3–5]. B7-H4 has approximately 25% homology in the extracellular portion with other B7-family members. Despite mRNA expression in various human tissues, immunohistochemistry analysis does not reveal positive staining for protein in any tissues or organs from healthy human individuals[6], indicating a tight control of B7-H4 protein expression, possibly at the posttranslational level. Cell-surface B7-H4 expression can be induced on human T cells, B cells, monocytes and Dendritic Cells(DCs) after in vitro stimulation. It is reported that B7-H4’s unidentified receptor is also expressed on activated T cells. In mice, B7-H4 was found to be expressed constitutively by B220+ B cells in the spleen and to be downregulated after activation[3]. B7-H4 protein is expressed in many cancers such as ovarian, breast and lung cancer[7]. B7-H4 may be responsible for the escape of tumor cells from tumor-specific T-cell responses in cancer patients. Immobilized B7-H4-immunoglobulin inhibited T-cell proliferation, prevented them from entering the cell cycle and reduced the secretion of interleukin (IL)-2, IL-4, IL-10 and interferon (IFN)-γ in response to CD3/CD80 stimulation. Together, these studies reveal an important role for B7-H4 as a coinhibitor of T-cell immunity.
In order to understand the costimulatory function of B7-H4 on cellular immunity, human B7-H4 gene was cloned and expressed in the E coli system, to analyze the role it plays in T-cell modulation in vitro. In this report, we first described methods for high-level expression and purification of the hB7-H4 extracellular domain as GST fusion protein from E coli. Further studies on the target protein GST/hB7-H4 indicated its biological function of inhibiting T-lymphocyte proliferation and cytokine secretion, which may be important evidence for the hB7-H4 down-regulatory feature.
Materials and methods
Materials The E coli strain TOP10 and expression host BL21-RIL was purchased originally from Novagen (La Jolla, CA, USA). Expression vector pGEX-5X-3 (GST fusion protein vector) was provided by Amersham Pharmacia (Piscata-way, NJ, USA). All restriction endonucleases, T4 DNA ligase, rTaq & Pyrobest DNA polymerase, pMD18-T Vector and DNA molecular weight marker were obtained from TaKaRa Biotech (Dalian, China). Protein molecular weight marker is a product of Fermentas (Burlington, Ontario, Canada). Gel Extraction Mini Kit, Plasmid Mini Kit, RT-PCR Mini Kit were purchased from Shanghai Watson Biotechnologies Inc (Shanghai, China). Anti-CD3 monoclonal antibody was from Sigma (St Louis, MO, USA). BM Chemiluminescence Western Blot Kit (Mouse/Rabbit) was obtained from Boehringer (Mannheim, Germany). IL-2 and IFN-γ ELISA kit were from R&D Systems (Minneapolis, MN, USA). Protein concentrations were estimated by the Bradford method using Bio-Rad protein assay. All other reagents and apparatus were of high quality available from commercial sources.
Cloning of hB7-H4 cDNA and construction of expression vector Using human cDNA FLJ22418 as a template, the full-length hB7-H4 cDNA was obtained by PCR. The PCR amplification was carried out with the following primers: B7-H4-A: 5'- ACG
Expression of GST/hB7-H4 fusion protein The E coli strain BL21-RIL, transferred with the recombinant vector pGEX-5X-3/hB7-H4, was cultured in 10 mL LB medium containing 100 µg/mL ampicillin and 2% glucose at 37 ℃ overnight. The next day, 2 mL overnight bacteria culture was added to 200 mL fresh medium, and incubated in two 500-mL conical flasks at 37 ℃, 225 r/min. To ensure adequate aeration, flasks were filled to only 20%–25% capacity. When the OD600 value reached 0.6−0.8, the recombinant protein was induced by the lactose analog isopropyl-β-D thiogalactoside (IPTG) to the final concentration of 1 mmol/L. Five hours later, cells were harvested by centrifugation and rinsed three times in 1×PBS.
Refolding and purification of the recombinant protein
Preparation of extracts to be identified by SDS-PAGE The expressed fusion protein was resuspended in 6 mL dual-boiling water (Add 1–10 mmol/L DTT (1,4-Dithiothreitol), 1 mmol/L PMSF (Phenylmethyl Sulfonyl Fluoride)). Adding DTT prior to cell lysis can significantly increase binding of some GST fusion proteins to Glutathione Sepharose. The addition of lysozyme (0.1 volume of a 10 mg/mL lysozyme solution in 25 mmol/L Tris-HCl, pH 8.0) may help to improve the efficiency of sonication. After being sonicated at a high setting (duty time=10 s, rest time=10 s) of 480 W in ice until the viscosity disappeared, the sample was centrifuged at 5000×g for 10 min. Cell disruption is evidenced by partial clearing of the suspension or may be checked by microscopic examination. Frothing was avoided as this may denature the fusion protein. Oversonication can also lead to co-purification of host proteins with the GST fusion protein[8]. The supernatant was saved and the pellet was resuspended in 12 mL washing buffer [20% (v/v) Glycerol, 2% (v/v) Triton X-100, 2 mol/L urea, 50 mmol/L NaCl, 50 mmol/L EDTA, 100 mmol/L Tris-Cl (pH 8.0)]. After 10 min at room temperature, the samples were centrifuged at 5 000×g for 10 min at 4 ℃. Following three cycles of washing, the precipitate was denatured in 8 mol/L urea with 50 mmol/L Tris-Cl, 100 mmol/L NaCl, 2 mmol/L EDTA, 5 mmol/L DTT, 1 mmol/L PMSF (pH 8.9) overnight at 4 ℃. The supernatant and deposit collected respectively by centrifugation for 15 min at 12 000×g were analyzed by SDS-PAGE (12%).
Purification by GST-affinity chromatography After high-speed centrifugation, the supernatant was dropped into 200 mL renaturation solution (1 mmol/L reduced and 0.2 mmol/L oxidized glutathione, 1 mol/L urea, 50 mmol/L Tris-Cl, 100 mmol/L NaCl, 1 mmol/L PMSF, 50 mmol/L EDTA, pH 8.0) with constant agitation at 4 ℃ for 48 h. The temperature of renaturation was then changed to room temperature. Another 12 h later, the renaturation solution containing the fusion protein was dialyzed for 24 h at 4 ℃ against 1×PBS (pH 7.3), which was replaced every 6 h. The renatured protein was then loaded onto the 1×PBS pre-equilibrated GST affinity chromatography column (Glutathione Sepharose 4B, Amersham Pharmacia) at a rate of 1 mL/min. Following another equilibration, the bound GST-fusion protein was then eluted with elution buffer (50 mmol/L Tris-HCl, 10 mmol/L reduced glutathione, pH 8.0). Decreasing the flow rate during purification or further elution with higher concentrations of glutathione (20–50 mmol/L) at times improved yield. The pooled fractions containing GST/hB7-H4 recombinant proteins were filtered through sterile 0.22 µm membrane. Protein concentration was determined by the Bradford method using bovine serum albumin as standard. The protein was also subjected to SDS-PAGE to determine the purity of the target protein[9–12].
Identification by SDS-PAGE and Western blot The total bacterial protein was loaded to sodium dodecyl sulfate-polyacrylamide (SDS) gel electrophoresis (12%), transferred onto the nitrocellulose filters, and stained with rabbit-anti human polyclonal antibodies against hB7-H4, which was prepared in our laboratory (data not shown). To eliminate non-specific reaction, polyclonal antibodies were cross-adsorbed with anti-GST antiserum with E coli proteins (BL21-RIL harboring GST-mock) using CNBr-activated Sepharose 4B[8]. The protein band was visualized by using the BM Chemiluminescence Western Blotting Kit according to the manufacturer’s instructions.
T-cell proliferation and cytokine assay Peripheral blood mononuclear cells were isolated from healthy peripheral human blood by Ficoll gradient centrifugation. Monocytes were removed by plastic adherence for 2 h in RPMI-1640 Medium (Roswell Park Memorial Institute)+10% fetal calf serum (FCS) at 37 ℃. CD3+ T-cells were collected by passing over a nylon wool column (>90%)[13]. For T-cell proliferation assay, a 96-well flat-bottom plate was precoated with CD3 mAb (0.3 µg/mL) in PBS at 4 ℃ overnight. Purified T cells were then seeded into a 96-well plate (2×104 /well) in the presence of various concentrations of GST/hB7-H4. The negative control was a corresponding dose of soluble GST (sGST) protein, which was prepared in the same way as GST/hB7-H4 with a high purity (>90%). After incubation for 96 h in a 37 ℃, 5% CO2 incubator, the supernatant of T cells was harvested immediately before the addition of [3H]thymidine.[3H]thymidine 1 µCi (1 Ci=37 GBq) was added to each well and the cells were incubated for another 16 h before harvesting. All the measurements were performed in triplicate[12]. The concentrations of IL-2 and IFN-γ in culture supernatant were detected with ELISA kits according to the manufacturer’s instructions.
Statistical analysis All data presented as mean±SD. Student’s t-test was used to determine the statistical significance. P<0.05 was considered statistically significant.
Results
Cloning and construction of expression vector of hB7-H4 With PCR, an 870 bp fragment was amplified from cDNA FLJ22418 (Figure 1A). Confirmed by DNA-sequencing (Bioasia, Shanghai, China), the obtained sequence was entirely identical to the published sequence of human B7-H4 in the Genbank Database (Accession N
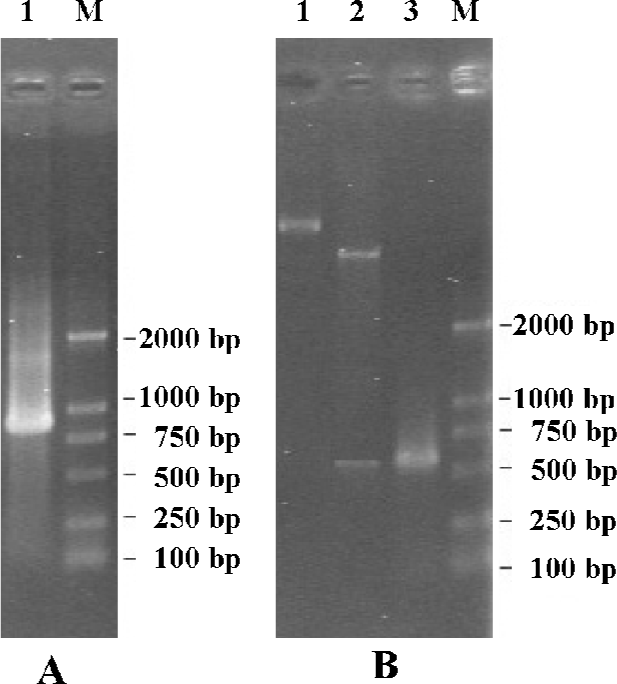
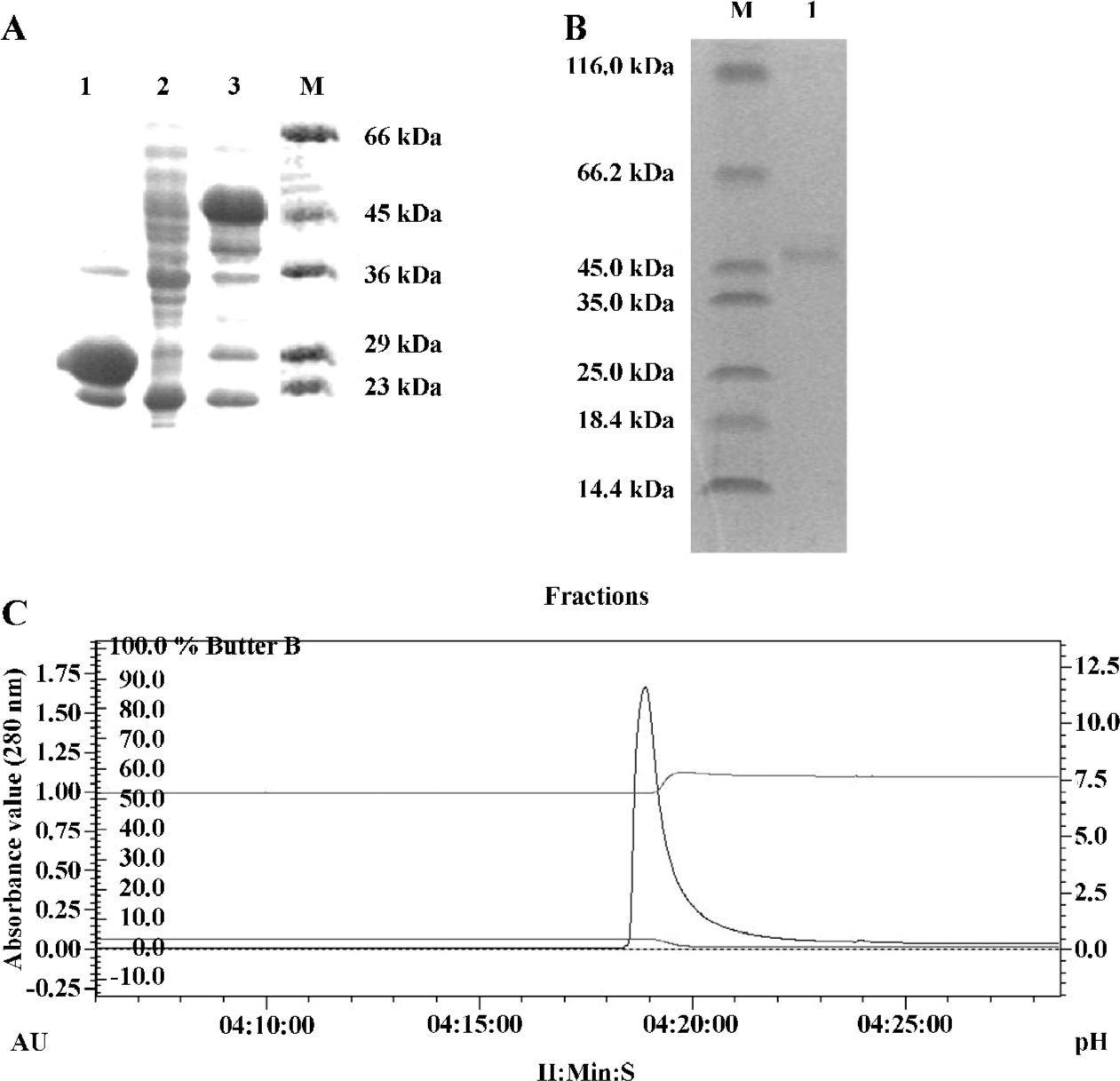
Expression and purification of recombinant protein GST/hB7-H4 After being transformed with pGEX-5x-3/hB7-H4, E coli BL21-RIL was induced to express fusion protein GST/hB7-H4. The electrophoretic mobility of the expressed protein approximated to a molecular weight of 21 kDa plus the 26 kDa GST protein, which is in agreement with the molecular weight 47 kDa calculated based on its deduced amino acid sequence. And the expressed protein constituted 52% of the total bacterial protein after being induced with 1 mmol/L IPTG for 5 h at 37 ℃ (Figure 2A). The insoluble protein following denaturation and renaturation was purified through GST affinity chromatography column. The elution profile was monitored and analysis by SDS-PAGE showed that the purity reached 95% (Figure 2B, C).
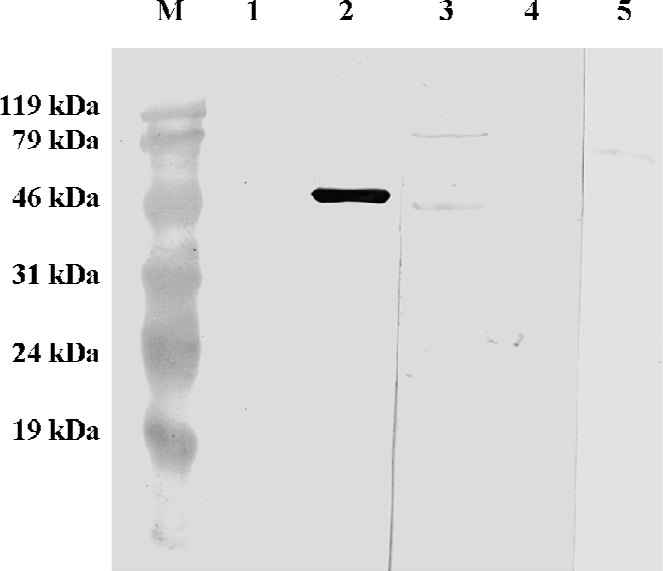
Fusion protein identified by Western blot With recombinant GST/hB7-H4 as antigen, Western blot showed that the polyclonal antibodies against hB7-H4 could recognize a protein with approximately 47 kDa, which is in accordance to the molecular weight of GST/hB7-H4. Lysates of uninduced BL21-RIL harboring GST/hB7-H4 and sGST protein respectively were set as negative control. As expected, no obvious band was detected. This result confirmed that the fusion protein had the same immunodomain as hB7-H4 (Figure 3).
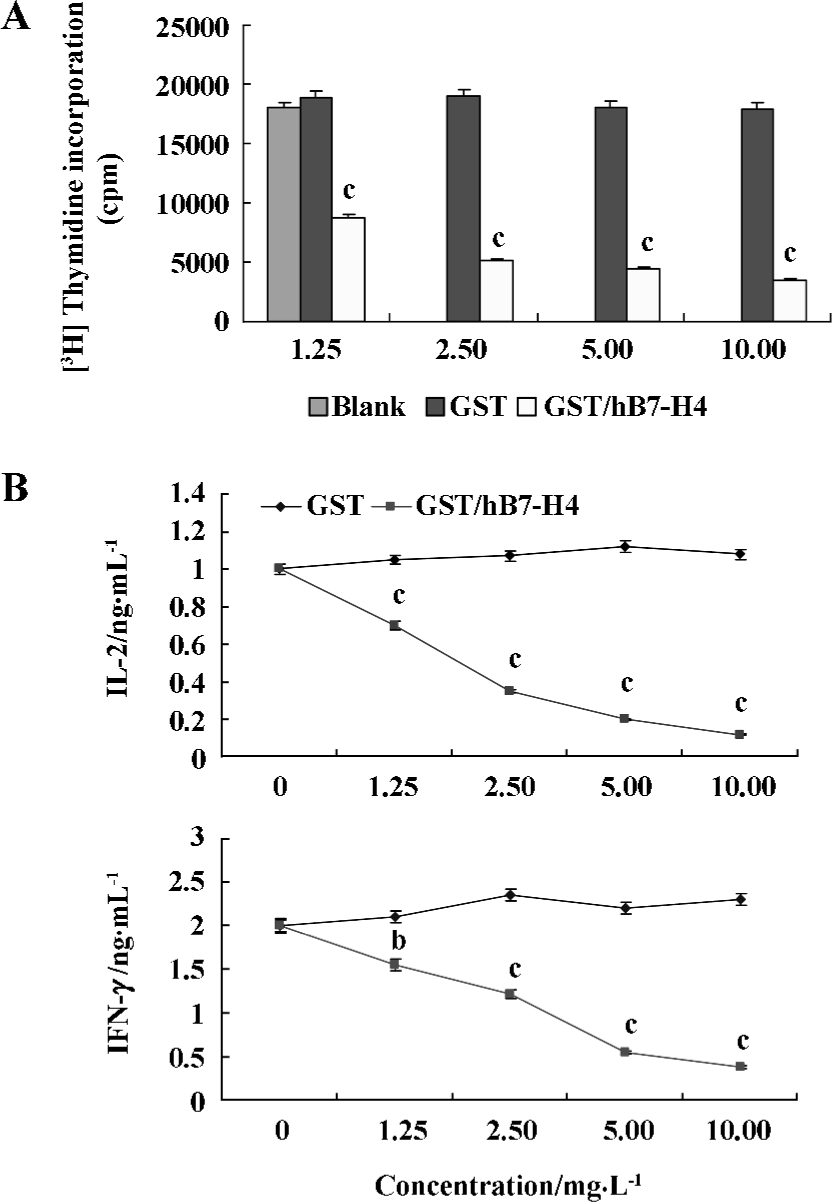
Inhibitory effects of T cells costimulated by GST/hB7-H4 in vitro The proliferation of T cells was determined by [3H]thymidine assay after a 96-h incubation. The results showed that GST/hB7-H4 protein could obviously inhibit CD3mAb activated T cell (0.3 µg/mL) (Figure 4A). At the same time, it reduced the production of IL-2 and IFN-γ of anti-CD3-stimulated T cells in a dose-dependent manner in vitro (P<0.05, unpaired t-test) (Figure 4B, C). These data were representative of three independent experiments.
Discussion
B7-H4, the latest member of the B7 superfamily, was only identified recently. Mounting data showed that hB7-H4 might represent a novel regulatory means for cell-mediated immunity. In our research, we found that immobilized B7-H4 could inhibit the proliferation of T cells experiencing Ag stimulation. In addition, B7-H4 was known to suppress the production of Th1-derived cytokines such as IL-2 and IFN-γ from the naive T cell. Preliminary data indicated that engagement by B7-H4 on receptors on activated T cells had a minimal effect on programmed cell death but had a profound effect on cell cycle arrest, by which most of the T cells activated by anti-CD3 are arrested in the G0/G1 phase[3–5]. Therefore, B7-H4 may play a role in the negative regulation of T-cell immunity in peripheral tissues.
The work presented in this paper demonstrated that hB7-H4 could be expressed in bacteria and readily purified in reasonable quantities for research on its biofunctions. In theory, the GST/hB7-H4 protein could be specially digested with thrombin to delete the GST part. But because of economic problems, we did not do so. Our results showed that the recombinant protein GST/hB7-H4 maintained the bioactivity to inhibit T-cell proliferation and IL-2 secretion. This indicated that we had a convenient tool to study hB7-H4 and its functions in regulating immune responses. The full-length cDNA of human B7-H4 cloned by PCR from cDNA fragments would facilitate the preparation of the hB7-H4 monoclonal antibody for further study. In our study, the efficiency of the recombinant protein expression was time- dependent reaching the highest level at the 5 h after adding IPTG. Meanwhile, we gained a lot of experience in purification and refolding of proteins. We first found GST/hB7-H4 protein could obviously reduce IL-2 secretion, which suggested hB7-H4 might have effect on T-cell-mediated immune response. Our results showed that the special antibody against hB7-H4 could partly reverse the inhibition of T cells mediated by GST/hB7-H4 fusion protein. The putative counter-receptor of hB7-H4 could be rapidly expressed on T cells after stimulation by PHA or anti-CD3 mAb for 6 h (data not shown).
In conclusion, our study demonstrated that the prokaryote expression system could be used to generate hB7-H4 protein with natural spatial conformations and biological functions, which provided an efficient and economical way for the preparation of this target protein, and received clues on how to clarify the mechanism of hB7-H4 in immune responses.
Acknowledgement
We Thank Dr Hiroko HATA (Laboratory of Genome Structure Analysis, Human Genome Center, Institute of Medical Science, University of Tokyo, Japan) for his generous gift of clone HRC08590, which contains full-length human B7-H4 cDNA.
References
- Baxter AG, Hodgkin PD. Activation rules: the two signal theories of immune activation. Nature Rev Immunol 2002;2:439-46.
- Chen LP. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nature Rev Immunol 2004;4:336-47.
- Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003;18:849-61.
- Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 2003;18:863-73.
- Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA 2003;100:10388-92.
- Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol 2003;171:4650-4.
- Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, et al. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Expr Cell Res 2005;306:128-41.
- GST Gene Fusion System Handbook. Amersham Biosciences 2002; p23.
- Kimiaki M, Mineyuki M, Keiichi K. Expression of a synthetic gene encoding human transthyretin in Escherichia coli. Protein Expr Purif 2003;30:55-61.
- Ayrapetov MK, Lee S, Sun G. Expression, purification, and biochemical characterization of Chk, a soluble protein tyrosine kinase. Protein Expr Purif 2003;29:148-55.
- Miroy G, Monteiro MJ. Expression and purification of a convenient Ca2+-calmoduli-dependent protein kinase II GST-fusion substrate. Protein Expr Purif 2002;26:343-8.
- Joseph S, David WR. Molecular Cloning: A Laboratory Manual.3rd Edition. New York: Cold Spring Harbor Laboratory Press; 2001.
- Sheng WH, Wang YL, Geng YP, Si L. Bacterially producted human B7-1 protein encompassing its complete extracellular domain maintains its costimulatory activity in vitro. Chin Med J 2000;173:714-9. Chinese..
