Anti-inflammatory effect of amurensin H on asthma-like reaction induced by allergen in sensitized mice1
Introduction
Asthma is a chronic, inflammatory disease of the airways affecting approximately 8% of the world’s population[1]. The disease is characterized by airway inflammation, increased mucus production, intermittent airway obstruction and hyper-responsiveness[2]. There are rising trends in asthma prevalence and severity, with accompanying increases in morbidity and mortality, especially among young children[3,4]. Despite decades of research, the therapy of this pathological condition has remained essentially unchanged[5]. In recent years, only few new drugs were proposed for the treatment of asthma, such as leukotriene receptor antagonists[6], anti-immunoglobulin E antibodies[7], and phosphodiesterase inhibitors[8]. There is still a compelling need for anti-asthma drug with high effectiveness and low side effects.
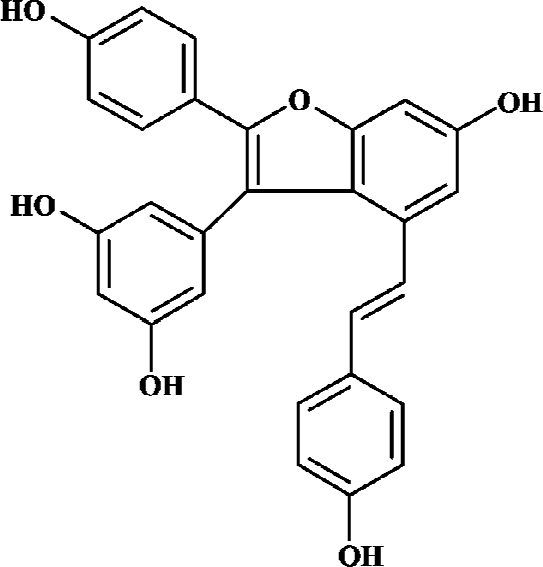
Amurensin H, a resveratrol dimer having a benzofuran moiety (structure as shown in Figure 1), is an alcoholic abstract of Vitis amurensis. Vitis amurensis Rupr, belonging to a family of Vitaceae, grows in northeast and central parts of China, whose roots and stems are used as Chinese folk medicine for many years. Amurensin H can be obtained scarcely as a natural product. A synthetic route has been achieved according to its biogenetic pathway, which provided enough amurensin H for testing its anti-inflammatory effects. Amurensin H has shown strong anti-inflammatory action in vitro and in vivo, for example, the production of tumor necrosis factor-α (TNF-α) and interleukin 8 (IL-8) in supernatants of LPS-stimulated HL-60 cell was reduced by amurensin H at the concentration of 100 nmol/L; the activation of nuclear factor-κB (NF-κB) in HL-60 cell was inhibited by amurensin H at the concentration of 100 nmol/L; the degranulation of mast cell mediated by compound 48/80 was inhibited by amurensin H at the concentration of 1 μmol/L. In two acute inflammatory models, mouse ear edema induced by croton oil and rat paw edema induced by carrageenan, amurensin H also showed significant inhibitory action (data not shown).
The purpose of the present study was to extend our findings of amurensin H to an in vivo mouse asthma model for further exploration of its anti-inflammatory action in an allergic condition. In this experiment, TNF-α, the pro-inflammatory cytokine, together with IL-4, IL-5, and IL-13, the T helper 2 cells (Th2 cells) cytokines, were evaluated. Inflammatory cell recruitment and lung pathology were also investigated.
Materials and methods
Materials Chemicals (analytical-grade) were purchased from Sigma-Aldrich Chemical (St Louis, MO, USA). The ELISA kits for IL-4, IL-5, and IL-13 were purchased from R&D Systems (Minneapolis, MN, USA). The ELISA kits for TNF-α were purchased from eBioscience Company (San Diego, CA, USA). Amurensin H was provided by Prof Mao LIN[9,10].
Animals and treatments Specified pathogen-free, male BALB/c mice 6–8 weeks of age (Experimental Animal Center, Chinese Academy of Medical Sciences and Peking Union Medical College, China) were divided into 6 groups. Each group consisted of 20 weight-matched animals. Group 1 was given 0.1 mL of saline (ip) on d 0 and d 14. On d 18–22, animals were challenged with saline for 20 min[11]. These animals were referred to as naïve animals. The remaining mice were sensitized by intraperitoneal injections of 20 µg OVA (grade V, Sigma) and 4 mg Al(OH)3 suspended in 0.1 mL of saline on d 0 and 14. On d 18–22, animals were challenged with an aerosol of 1% OVA suspended in saline for 20 min. Group 2 was given vehicle (10% ethanol solution, v/v) orally and referred to as the vehicle group. Group 3 was given 1 mg/kg of dexamethasone (Dexa, po) 1 h before each OVA aerosol challenge. This group was referred to as positive control. Group 4, 5 and 6 were orally administered amurensin H at the doses of 49, 70, and 100 mg/kg (suspended in 10% ethanol solution) respectively from d 15 to the last day. Animal experiments were performed according to the Institutional Guidelines for Animal Care and Use of Chinese Academy of Medical Sciences and Peking Union Medical College, in agreement with the Good Laboratory Practice Rules.
Bronchoalveolar lavage fluid (BALF) 24 h or 48 h after the last aerosol OVA challenge, mice were killed with an overdose of sodium pentobarbital (200 mg/kg, ip). Each group was divided into 2 subgroups with 10 mice in each group. The two subgroups were used for preparation of BALF at different time points.
To obtain BALF, the lungs were lavaged with 0.6 mL of ice-cold phosphate balance solution (PBS). BALF was centrifuged at 100×g for 15 min at 4 °C. The supernatant was decanted and stored at -80 °C for later analysis. The cell pellet was resuspended in 1 mL PBS. Total BALF cell counts were obtained using a hemocytometer cytospin (Shandon, Pittsburgh, PA, USA). The BALF cells were stained on glass slides with modified Wright-Giemsa stain. Differential cell counts of macrophages, lymphocytes, neutrophils, and eosinophils were performed in 200 cells per slide (one slide per animal).
Cytokines The concentrations of TNF-α, IL-4, IL-5 and IL-13 in the BALF were determined by ELISA kits. The limits of detection were as follows: TNF-α, 8 pg/mL; IL-4, 2 pg/mL; IL-5, 7 pg/mL; IL-13, 1.5 pg/mL.
Histologic examination 48 h after the last aerosol OVA challenge, lung tissues were extracted and fixed in 10% neutral formalin and stored at 4 °C for later processing. The tissue was washed, dehydrated and embedded in paraffin. Tissue sections of 6-mm thickness were stained with Mayer’s hematoxylin and eosin (H&E) for assessment of tissue damage and mucus production. H&E stained sections were examined by bright field microscopy and images captured with an Olympus DP1T digital camera system (Olympus Optical, Tokyo, Japan).
Statistical analysis Data were presented as mean±SD. Student’s t-test was used to determine significant differences between the treatment group and vehicle group. The critical level for significance was set at P<0.05.
Results
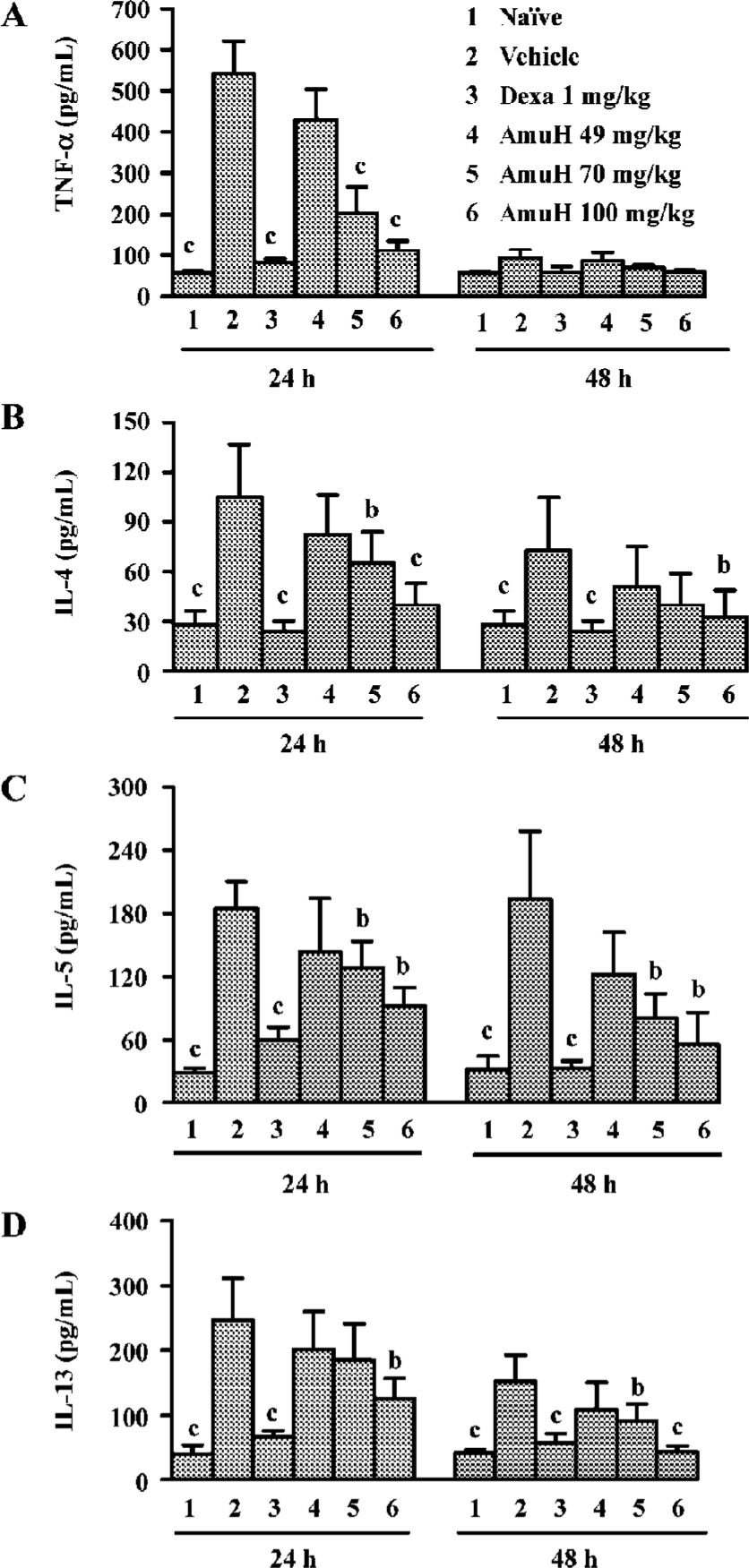
Effects of amurensin H on TNF-α, IL-4, IL-5, and IL-13 levels in BALF The level of TNF-α in BALF increased at 24 h post last OVA-challenge (P<0.05 vs naïve mice, Figure 2A) and Dexa reduced the increase of TNF-α (P<0.05 vs vehicle group). Oral administration of amurensin H before challenges decreased the level of TNF-α at 24 h (P<0.05 vs vehicle group). The level of IL-4 also increased at 24 h post last OVA-challenge (P<0.05 vs naïve mice, Figure 2B) and Dexa reduced the increase of IL-4 at 24 h (P<0.05 vs vehicle group). Oral administration of amurensin H before challenges significantly decreased the level of IL-4 at 24 h (P<0.05 vs vehicle group). The increase of IL-4 in the vehicle group and the decrease of IL-4 in Dexa and amurensin H treated groups were also observed at 48 h, though not to a statistically significant degree. The level of IL-5 and IL-13 both increased at 24 h (P<0.05 vs naïve mice) or 48 h (P<0.05 vs naïve mice, Figure 2C, 2D) after the last OVA-challenge. Dexa reduced the increase of IL-5 and IL-13 at both time points (P<0.05 vs vehicle group). Oral administration of amurensin H before challenges decreased the level of these two cytokines both at 24 h (P<0.05 vs vehicle group) and at 48 h (P<0.05 vs vehicle group). There was a similar trend towards the change of cytokine to different time points, that is, the levels of TNF-α, IL-4, IL-5 and IL-13 were higher at 24 h than they were at 48 h.
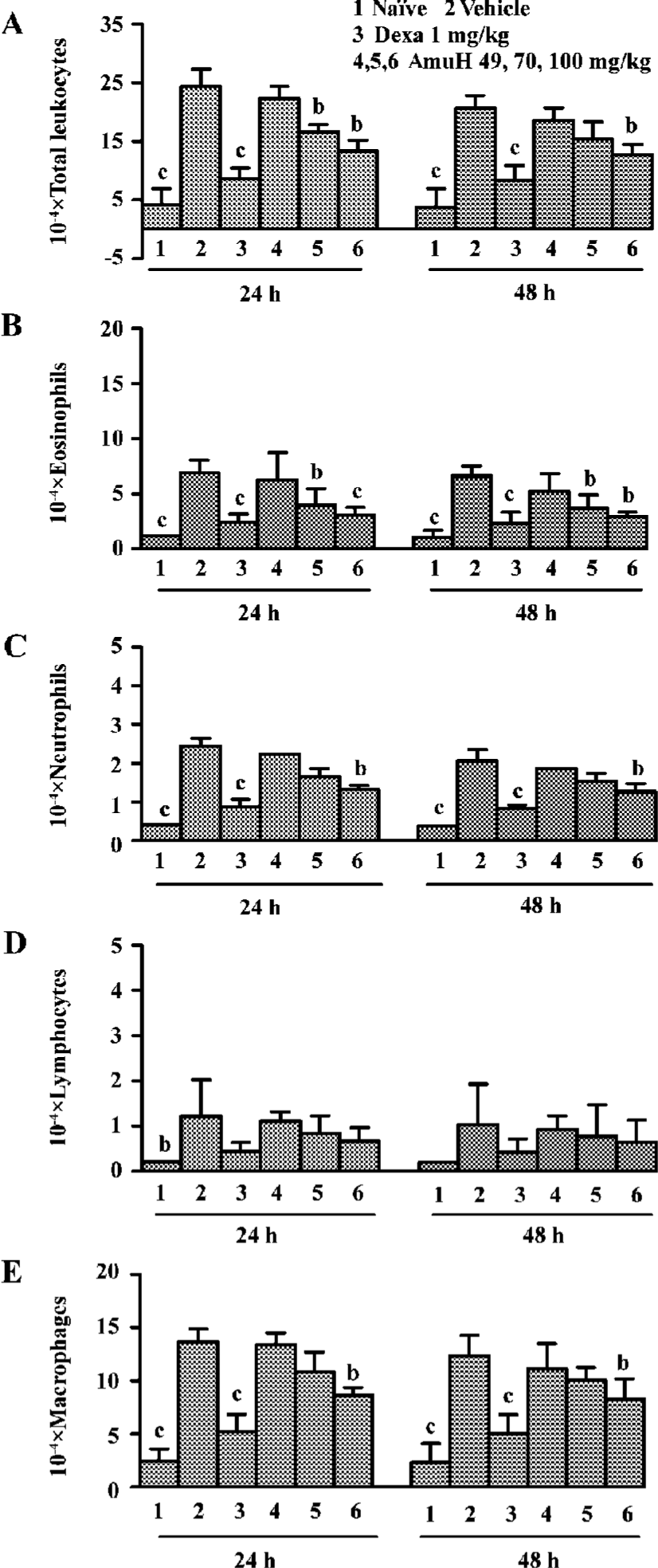
Effects of amurensin H on OVA-induced inflammatory cell recruitment in BALF BALF was collected 24 h and 48 h after the last OVA aerosol challenge. Total and differential cell counts were performed. OVA aerosol challenge induced a more than 5-fold increase in total leukocytes, which was comprised of increases in macrophages, eosinophils, neutrophils and lymphocytes (P<0.05 vs naïve mice, Figure 3A). There was an increased percentage and absolute number of eosinophils, neutrophils, lymphocytes, and decreased percentage of macrophages in the BALF of the vehicle group. Dexa at the dose of 1 mg/kg inhibited the infiltration of total leukocytes, macrophages, neutrophils, eosinophils, and lymphocytes (P<0.05 vs vehicle group). Amurensin H substantially reduced the number of total cells (P<0.05 vs vehicle group), which was mainly due to the significant reduction in eosinophils (P<0.05, P<0.01 vs vehicle group, Figure 3B). The numbers of lymphocytes and macrophages were also reduced a little in amurensin H-treated mice (Figure 3D, 3E). OVA challenge induced a rapid infiltration of neutrophils in BALF. As expected, this early neutrophilia was followed by later infiltration of lymphocytes and eosinophils (Figure 3B,3C,3D). The number of total inflammatory cells in the treatment group was lower at 48 h than it was at 24 h, which meant the recruitment of leukocytes was related to the levels of inflammatory cytokines, especially IL-5.
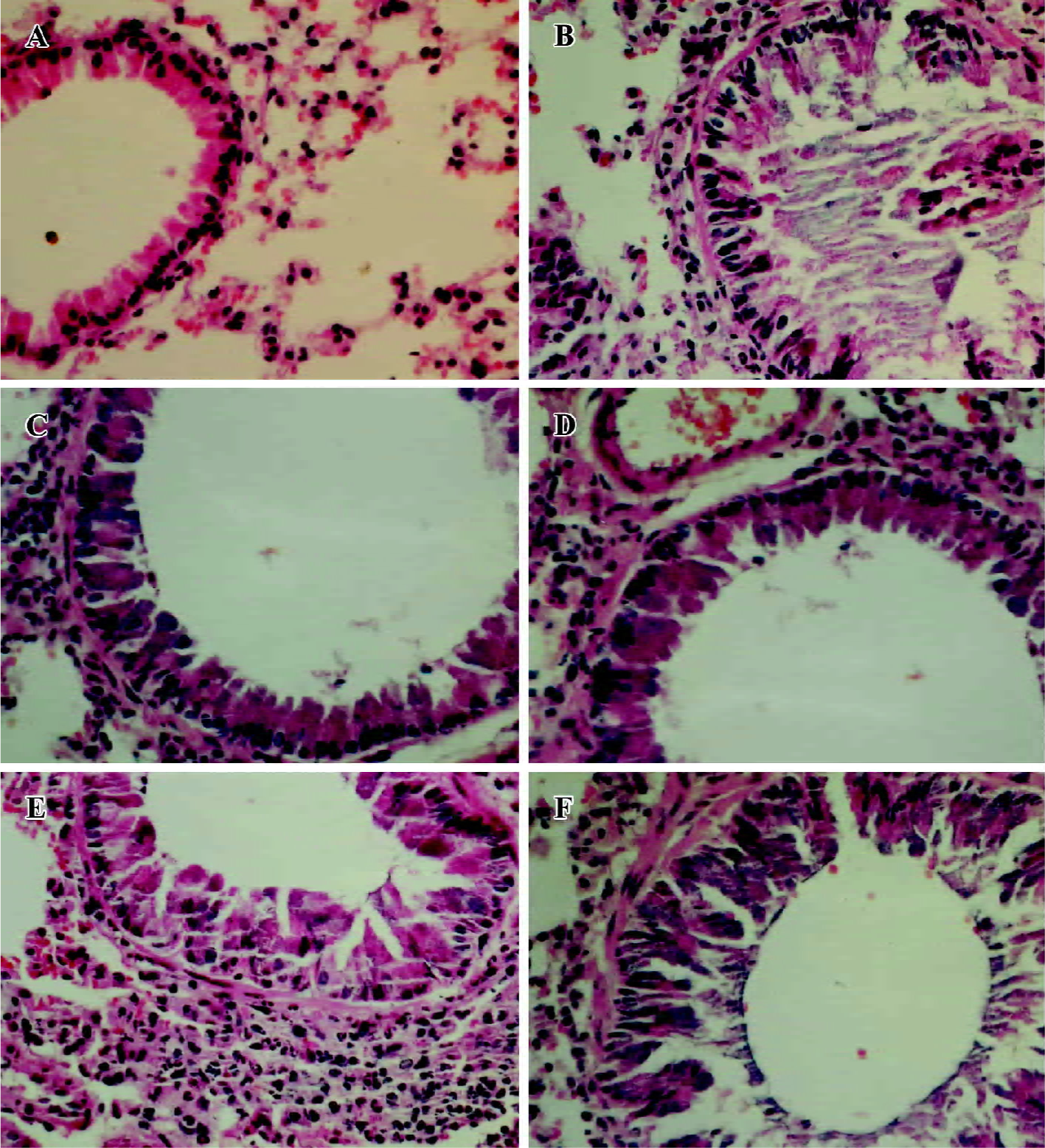
Effects of amurensin H on OVA-induced tissue damage and mucus production Lung tissues were collected 48 h after the last OVA challenge. OVA aerosol challenge induced a severe inflammatory reaction compared with naïve mice, characterized by mucus production, hyperemia, interstitial edema, inflammatory cell infiltration, and desquamation of bronchial epithelial (Figure 4B). Five times OVA challenge were performed in this experiment, so the inflammatory reaction was severer than those challenged 3 times in some other reports. Dexa (1 mg/kg, Figure 4C) or amurensin H (100 mg/kg, Figure 4D) treatments attenuated the mucus production and desquamation of bronchial epithelial as compared to the vehicle group. Amurensin H at a dose of 70 or 49 mg/kg showed attenuation in mucus production, but the desquamation of bronchial epithelial was not ameliorated.
Discussion
Current asthma therapies focus on two aspects of the disease, alleviating the symptomatic bronchoconstriction through relaxation of smooth muscle, and controlling the underlying pathology of inflammation. Bronchodilators are primarily used to reverse an ongoing bronchoconstriction. Their effects on inflammation are modest and usually require co-therapy with anti-inflammatory compounds[12-15]. The ability to modify the disease by breaking the cycle of inflammation is crucial for the successful treatment of asthma. The anti-inflammatory actions of amurensin H were strongly supported in this murine asthma model. Amurensin H could attenuate OVA-induced pulmonary inflammation, decrease production and release of pro-inflammatory cytokines into the airway. Although amurensin H was not as efficacious as Dexa in this preclinical model, corticosteroids have significant side effects, which cause concern over their use at high doses or over the long term, especially in children and the elderly[16]. In present study, the recruitment of leukocytes in BALF was studied at 24 h and 48 h post last challenge. The total numbers of infiltrated leukocytes increased significantly at both time points. Amurensin H generally did not show selectivity with respect to cell type in its inhibition of leukocyte accumulation in the BALF. Eosinophils, together with macrophages and lymphocytes were all significantly reduced by amurensin H.
Allergic asthma is characterized as a Th2 driven, IgE mediated disease with clear functional symptoms and underlying lung inflammation[1]. Th2 lymphocytes have a pro-inflammatory role by expressing and generating cytokines that attract and activate effect cells, leading to further cytokine release in allergic asthma[17]. The Th2-derived cytokines IL-4, IL-5, and IL-13 were investigated in this study. The time of lavage post challenge was chosen to coincide with the neutrophil peak (24 h), and eosinophil and lymphocyte peak (48 h) according to Kasserra et al’s report[1]. The levels of IL-4 and IL-13 increased significantly at 24 h post the last challenge. The levels of IL-4 decreased almost to baseline at 48 h post the last challenge. The level of IL-5 increased significantly in vehicle-treated group and this increase lasted to 48 h, when the level of IL-5 was even higher than it was at 24 h post the last challenge. Considering the critical effects of IL-5 on eosinophil differentiation, maturation, recruitment and activation[18], the increase of IL-5 may partly explain the later peak of eosinophils. TNF-α is believed to amplify inflammatory reaction as a pro-inflammatory cytokine. In this study, the level of TNF-α increased significantly at the first 24 h and decreased dramatically at 48 h post last challenge. The parallel change of TNF-α or neutrophils at different times may not be a simple coincidence, since neutrophil recruitment is demonstrated to be partly through the TNF-α pathway. Actually, the mechanisms responsible for the reaction of asthma are not fully understood and evidence points to a complex cascade of cell infiltration and mediator release from multiple cell types. The precise relationship between inflammatory cells and cytokines in this model is unclear. As mentioned previously, there exists a cycle of inflammation during the disease progress, and amurensin H seems to have the ability to break certain steps in this inflammation cycle. The expression of inflammatory cytokines is regulated by transcription factors and DNA-binding proteins. The activation of NF-κB has been reported in the OVA-induced asthma model. The inhibition of NF-κB activity by amurensin H was observed in an LPS induced HL-60 cell in our previous studies (submitted). This may partly explain the reason for its anti-inflammatory effects.
In conclusion, oral administration of amurensin H significantly reduced the OVA-induced increase of TNF-α, Th2 cytokines in BALF of sensitized mice. Amurensin H also inhibited inflammatory cells recruitment and infiltration in BALF of this model, suggesting a potential anti-allergic inflammation effect of this compound. Since the model employed in this study is exposed to amurensin H prior to antigen challenge, the result may suggest a prophylactic action of this compound. Further studies are needed to identify its molecular mechanism of anti-inflammatory action. Its anti-allergy action will be explored on some other asthma models with different species.
Acknowledgements
We are grateful to Prof Nai-kun ZHAO and Ms Rong ZHENG for the morphometric analysis.
References
- Kasserra CE, Harris P, Stenton GR, Abraham W, Langlands JM. IPL576,092, a novel anti-inflammatory compound, inhibits leukocyte infiltration and changes in lung function in response to allergen challenge. Pulm Pharmacol Ther 2004;17:309-18.
- Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest 2003;111:291-7.
- Frew AJ. Advances in environmental and occupational diseases 2004. J Allergy Clin Immunol 2005;115:1197-202.
- Hartert TV, Peebles RS Jr. Epidemiology of asthma: the year in review. Curr Opin Pulm Med 2000;6:4-9.
- Masini E, Vannacci A, Giannini L, Befani O, Nistri S, Mateescu MA, et al. Effect of a plant histaminase on asthma like reaction induced by inhaled antigen in sensitized guinea pig. Eur J Pharmacol 2004;502:253-64.
- Drazen JM. Asthma therapy with agents preventing leukotriene synthesis or action. Proc Assoc Am Physicians 1999;111:547-59.
- Spina D, Page CP. Asthma – a need for a rethink? Trends Pharmacol Sci 2002;23:311-5.
- Sullivan SD, Meltzer EO. Emerging therapeutic strategies for asthma management. J Manag Care Pharm 2003;9:14-21.
- Huang KS, Lin M, Cheng GF. Anti-inflammatory tetramers of resveratrol from the roots of Vitis amurensis and the conformations of the seven-membered ring in some oligostilbenes. Phytochemistry 2001;58:357-62.
- Huang KS, Lin M, Yu LN, Kong M. Four novel oligostilbenes from the roots of Vitis amurensis. Tetrahedron 2000;56:1321-9.
- Duan W, Aguinaldo Datiles AMK, Leung BP, Vlahos CJ, Wong WSF. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Inter Immunopharmacol 2005;5:495-502.
- Duan W, Chan H, Vlahos C, Wong W. Anti-inflammatory effects of LY294002, a PI3K inhibitor, in a mouse model of asthma. J Allergy Clin Immunol 2004;113:S219.
- Roh GS, Seo SW, Yeo S, Lee JM, Choi JW, Kim E, et al. Efficacy of a traditional Korean medicine, Chung-Sang-Bo-Ha-Tang, in a murine model of chronic asthma. Inter Immunopharmacol 2005;5:427-36.
- McIvor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. Am J Respir Crit Care Med 1998;158:924-30.
- Sears MR. Asthma treatment: inhaled beta-agonists. Can Respir J 1998; 5 Suppl A: 54–9.
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med 1999;159:941-55.
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 2003;111:450-63.
- Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med 2005;11:148-52.