Inhibition of ethanol-induced toxicity by tanshinone IIA in PC12 cells1
Introduction
Alcohol abuse is associated with several neurological disorders[1–3]. It is also closely associated with the pathogenesis of several neurological diseases such as Korsakoff’s Syndrome, Marchiafava-Bignami disease, pellagrous encephalopathy, and acquired hepatocerebral degenera-tion[4,5]. In addition, there is evidence that the brains of alcoholics undergo toxic changes including a reduction in brain volume[6–8], which may be due to the loss of neurons, shrinkage of neuronal cell bodies, or the reduction in the number and extent of dendrites. Recent studies have also shown that apoptosis is one of the main changes of neurodegenera-tion induced by ethanol[9–11]. All these changes may result from the reduction of endogenous antioxidant levels, the formation of reactive oxygen species (ROS), the depletion of GSH, and the DNA fragmentation induced by ethanol exposure[12,13]. Thus, it is important to find a therapeutic drug that can prevent ethanol-induced neurotoxicity.
Tanshinone (Tan), a major active ingredient of Salvia miltiorrhiza extract, is a mixture of many kinds of analogue compounds[14]. Tan has been reported to have protective effects on neuron cells in some in vitro models including hypoxia, hypoglucose, oxidant injury, calcium overload, nitric oxide neurotoxicity, and glutamic acid injury[15]. Tan IIA is the most abundant and structurally representative of Tan and can reduce the brain infarct volume in transient focal cerebral ischemia in mice[16]. It has been reported that miltir-one inhibits upregulation of the GAGA receptor alpha4 subunit mRNA by ethanol withdrawal in hippocampus neurons[17]. In addition, recently published research suggests that Tan IIA inhibits serum deprivation-induced apoptosis in PC12 cells[18]. However, whether Tan IIA can protect against ethanol-induced neurotoxicity is still unknown.
The present study was designed to investigate whether Tan IIA can protect PC12 cells from the neurotoxicity induced by ethanol. For this objective, we analyzed the cell survival, ROS formation, lactate dehydrogenase (LDH) release, cell apoptosis, and levels of the apoptosis-related p53 protein expression in PC12 cells and explored the role of Tan IIA as a neuroprotective drug.
Materials and methods
Materials Tan IIA, a gift from Dr Xiao-yan YANG (Department of Clinical Pharmacology, Wuhan Tongji Medical College), was dissolved in dimethylsulfoxide (Me2SO). The concentration of Me2SO in the final culture media was ≤0.1% (v/v), and had no toxic effect on PC12 cells. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Fluka, and Dulbeco’s modified Eagle’s medium was from Invitrogen (Carlsbad, California, USA). The annexin V/propidium iodide (PI) kit was purchased from the Becton Dickinson (BD) Company (Rahway, New Jersey, USA).
Cell culture and ethanol treatment PC12 cells (American Type Culture Collection, Rockville, MD, USA) were cultured in DMEM medium supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum (FBS), 100 kU/L of penicil-lin, and 100 mg/L of streptomycin. They were split at a 1:3 ratio every 4 d, for up to 8 passages. The cells were subjected to the following conditions: (1) control (C, normal culture media alone); (2) ethanol (E, the medium supplemented with 50, 100, or 150 mmol/L ethanol); (3) Tan IIA (T, medium supplemented with 10 µmol/L Tan IIA); and (4) ethanol+Tan IIA (E+T. medium supplemented with 150 mmol/L ethanol and 10 µmol/L Tan IIA). The concentrations of ethanol were used as previously described[19–21]. Tan IIA was added to the media 4 h before adding ethanol at 70% confluence of PC12 cells. To maintain constant concentrations of ethanol in the experimental treatment, all cultures were placed in plastic containers with tight-fitting lids. All cellular analysis procedures were performed 24 h after the experimental conditions.
MTT cell viability assay Cell viability was measured using MTT assay. Briefly, the MTT solution (5 g/L) was added to each well and incubated at 37 ºC for 4 h. The culture medium was removed and 100 µL dimethyl sulfoxide was added to each well to dissolve the formazan. The optical density of each well was measured at 570 nm using a microplate reader (Spectra MAX 340, Molecular Devices Co, Sunnyvale, California, USA). One culture dish with PC12 cells was used for 1 experiment condition. Thus, each plate contained multiple wells of a given experimental condition and multiple control wells. This procedure was replicated for 2–4 plates/condition. The MTT data were converted to the percentage of the respective controls prior to analysis.
LDH release assay Following incubation, the media were analyzed for LDH activity using a modification of the calorimetric method[22]. Briefly, each cell culture supernate was added to fresh assay mixture (pyruvate substrate, NADH, Sigma color reagent, and 8 mol/L NaOH), and absorbance at 450 nm was recorded. The values were expressed as the sample mean absorbance normalized to the percentage of the control value.
Measurement of intracellular ROS formation The dye 2',7'-Dichlorodihydrofluorescein diacetate (DCFH2-DA), which is oxidized to fluorescent 2',7'-dichlorofluorescin (DCF) by hydroperoxides, was used to measure relative levels of cellular peroxides. The treated PC12 cells were washed, suspended in serum-free DMEM, incubated with 50 mmol/L dye at 37 °C for 30 min, washed with PBS, centrifuged, and the medium was then removed. Cells were dissolved with 1% Triton X-100 (Zhongshan Biotech, Beijing, China) and fluorescence was measured at an excitation wavelength of 485 nm, and an emission wavelength of 530 nm using a fluorescence microplate reader. The values were expressed as the sample mean absorbance normalized to the percentage of the control value.
Annexin V/PI staining After treatment, the cells were washed in cold PBS 3 times and resuspended in binding buffer (containing 10 mmol/L HEPES/NaOH pH 7.4, 140 mmol/L NaCl, 2.5 mmol/L CaCl2). FITC-annexin V was added to a final concentration of 1 mg/L and the cells were incubated in darkness for 10 min. The cells were then washed again in PBS, centrifuged and resuspended in binding buffer (100 µL). PI was added at a final concentration of 2 mg/L and incubated for 5 min before immediate analysis of the cells on the flow cytometer (Becton Dickinson, Rahway, New Jersey, USA). Viable cells were negative for both PI and annexin V; apoptosis cells were positive for annexin V and negative for PI, whereas late apoptotic cells displayed both high annexin V and PI labeling. Non-viable cells which underwent necrosis were positive for PI and negative for annexin V.
Indirect immuno-fluorescence After the PC12 cells were treated with ethanol or Tan IIA for 24 h, they were fixed in ethanol-acetone (1:1) for 15 min at 4 °C. After rinsing with phosphate-buffer saline, the cells were incubated with rabbit anti-rat p53 antibody (Santa Cruz, California,USA) at 4 °C for 12 h. FITC-labeled goat anti-rabbit antibody (Zhongshan Biotech, Beijing, China) was then added and further incubated for 45 min at 37 °C. Negative control was made by replacing the first antibody with phosphate-buffer saline. After the slides were covered with glycerol buffer, the expression of p53 was observed under fluorescence microscopy and photos were taken. The number of PC12 cells stained with p53 antibody was determined by counting the number of positive-stained cells in a total of at least 600 cells under magnification of ×400 in each group. Data from the 5 experiments were expressed as mean±SEM.
Flow cytometry The p53 protein expression was measured by flow cytometric analysis as previously describ-ed[23,24]. Briefly, the cells were harvested and washed in PBS. The cells were then fixed in 1.0% paraformaldehyde for 15 min and permeated with 0.2% Triton X-100. After washing, aliquots of 1×106 cells/mL were incubated at 37 °C for 30 min with rabbit anti-rat p53 (1:1000 dilution, Santa Cruz, California, USA). After washing with PBS, FITC-labeled goat anti-rabbit IgG antibody (Zhongshan Biotech, Beijing, China) was added and incubated for 45 min. Following this, 1×106 cells in each group were subjected to a flow cytometric analysis. Quantitative changes in p53 expression were assessed by mean fluorescence intensity (MFI). To correct for non-specific binding, PBS solution (instead of the first antibody) was added to the blank control tube.
Statistical analysis All data were presented as mean± SEM. One-way analysis of variance followed by the Q test using SPSS 11.0 Software (Chicago, Illinois, USA) was performed to determine statistical significance. P<0.05 was considered statistically significant.
Results
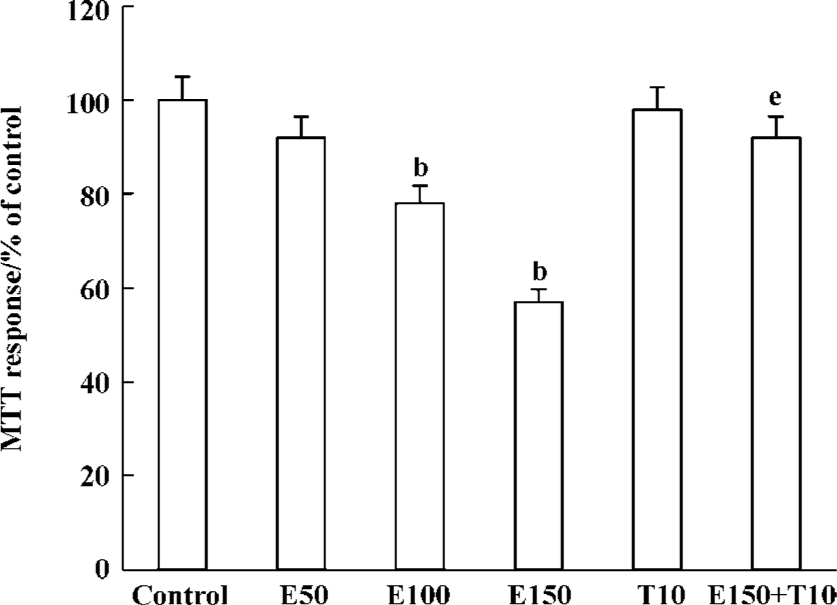
Effects of Tan IIA on the cell viability induced by ethanol The optical density in the control cultures exceeded that in the cultures containing 50, 100, and 150 mmol/L ethanol, which suggested that ethanol significantly induced the death of PC12 cells by MTT analysis (P<0.05). Tan IIA alone did not influence the survival rate of PC12 cells (P>0.05), but the exposure of PC12 cells to Tan IIA for 4 h before the addition of 150 mmol/L ethanol significantly increased PC12 cell viability (P<0.05, Figure 1).
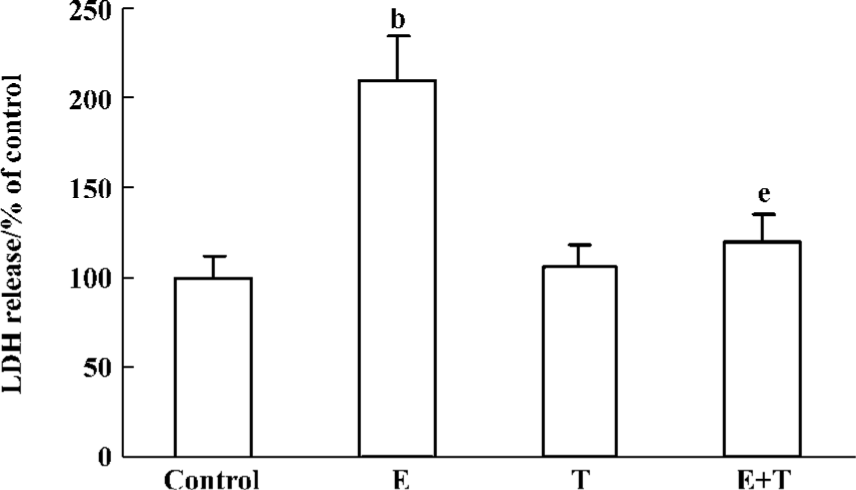
Necrosis always results in the disruption of the cytoplasmic membrane, and the necrotic cells release cytoplasmic LDH and other cell contents into the medium. We therefore examined the presence of LDH in the cultured medium. Ethanol induced an increased release of LDH, while Tan IIA attenuated these effects significantly (Figure 2).
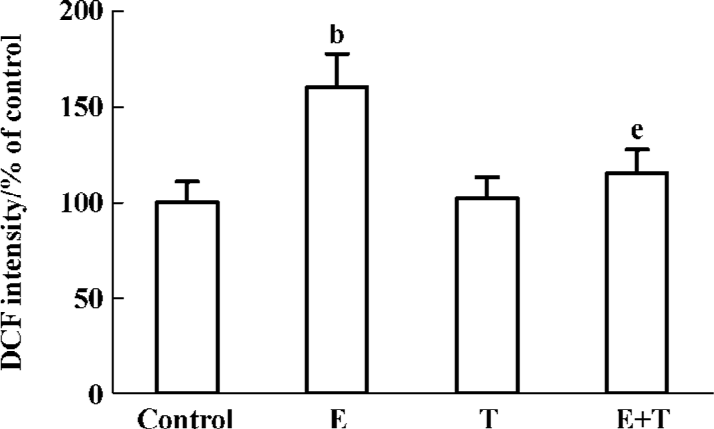
Effects of Tan IIA on ethanol-induced intracellular ROS formation After treatment with Tan IIA for 4 h, ethanol was added the culture media at a final concentration 150 mmol/L. The final DCF concentration significantly increased in the ethanol-treated cells. However, Tan IIA significantly suppressed DCF fluorescence intensity in the ethanol-exposed cells (P<0.05; Figure 3).
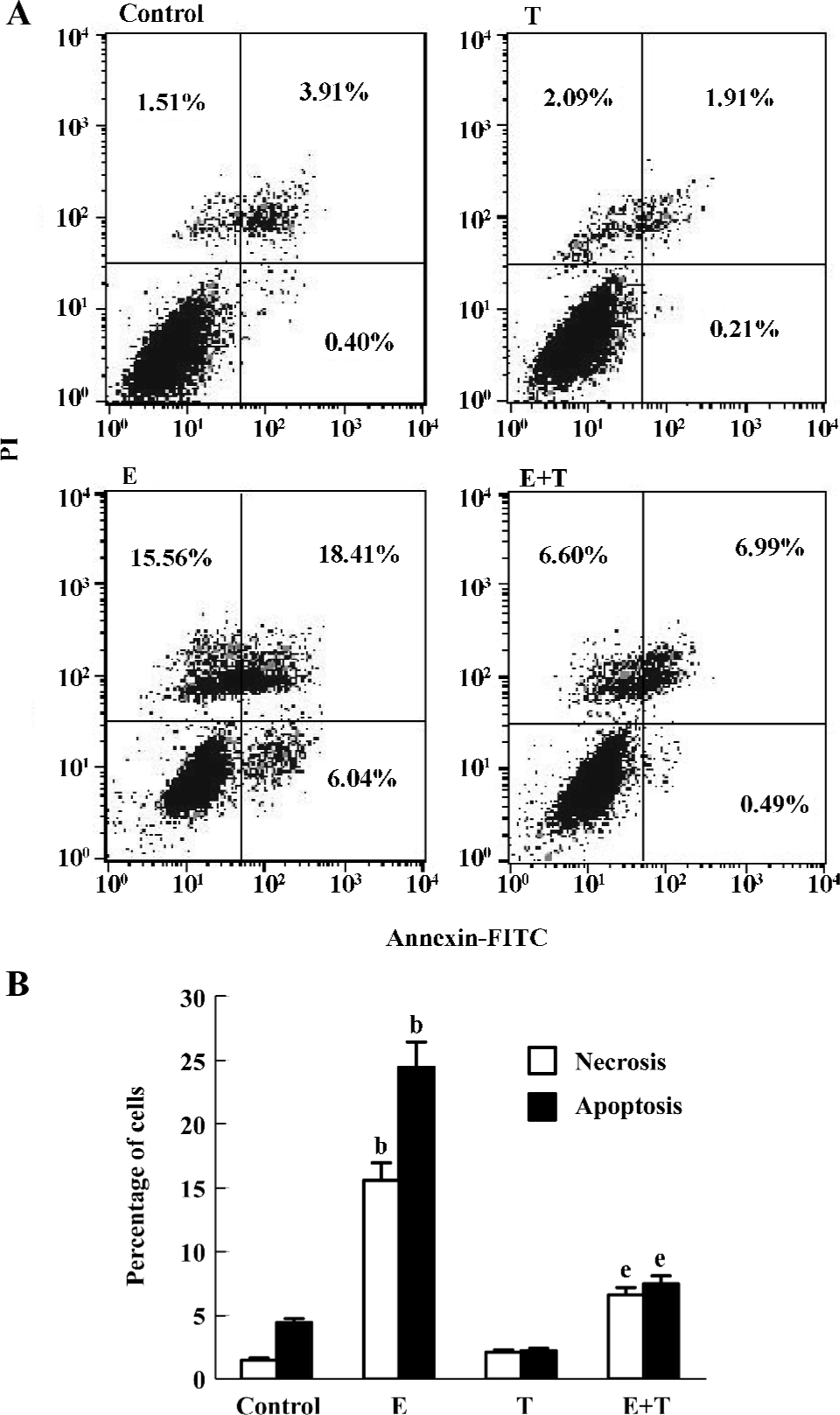
Effects of Tan IIA on ethanol-induced apoptosis Treatment with 150 mmol/L ethanol for 24 h resulted in an increase of the annexin V–/PI+, annexin V+/PI– and annexin V+/PI+ cells. Treatment with Tan IIA for 4 h decreased the number of annexin V–/PI+, annexin V+/PI– and annexin V+/PI+ cells, as compared with the control cells (P<0.05; Figure 4).
Effects of Tan IIA on ethanol-induced p53 expression Under fluorescence microscopy, almost no cell was stained with p53 under normal culture condition or when treated with Tan IIA alone. After the PC12 cells were cultured in medium containing 150 mmol/L ethanol, the percentage of p53 positive-stained cells was upregulated markedly (P<0.05). Having been pretreated with Tan IIA before adding ethanol, the ratio of the p53 positive-stained cells significantly decreased (P<0.05; Figure 5A).
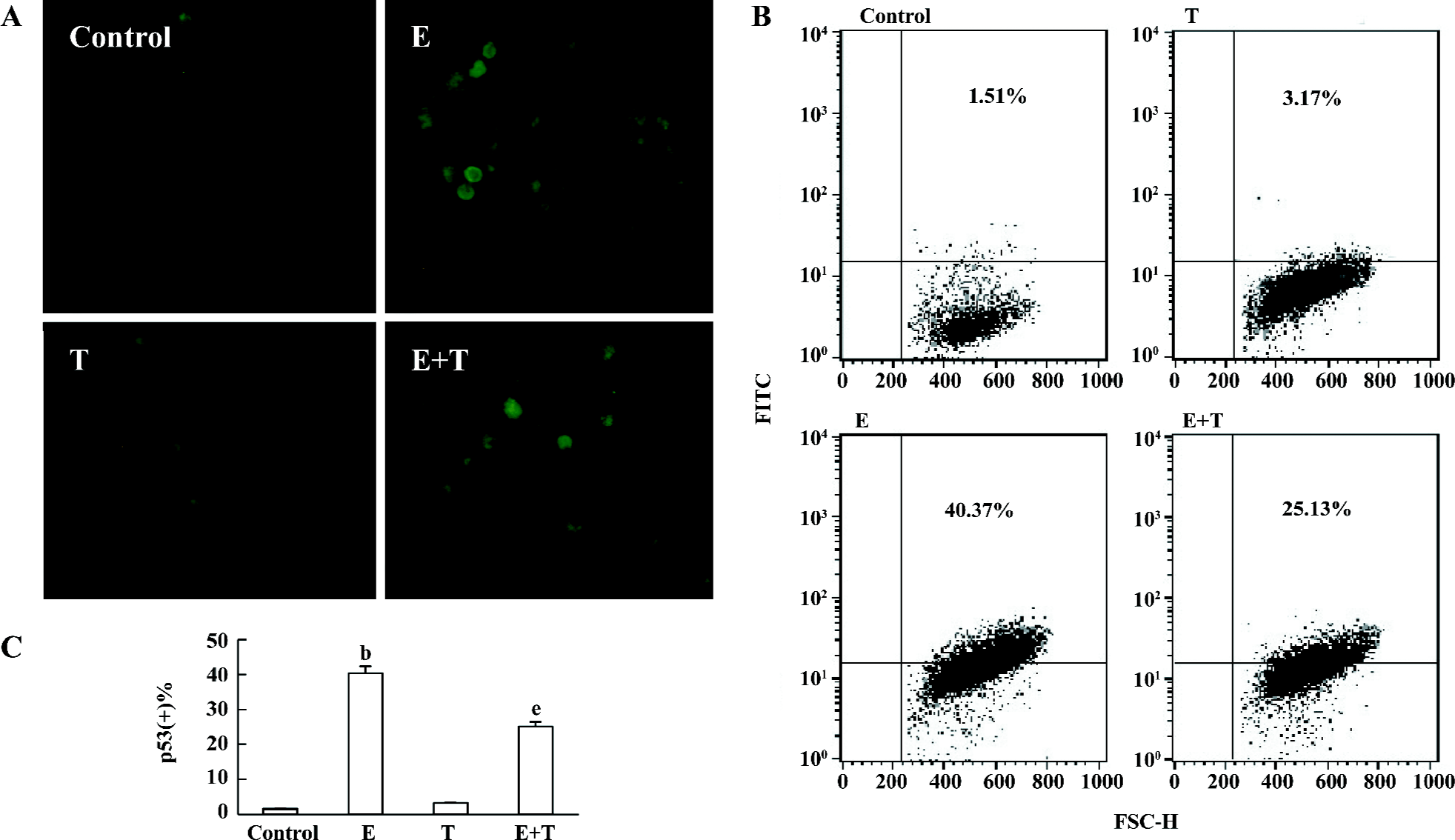
As shown by flow cytometry, there was no difference in p53 protein expression between the control and the Tan IIA-treated PC12 cells. A significant increase in the p53 protein expression was observed when the cells were treated with 150 mmol/L ethanol, which was significantly attenuated by Tan IIA pretreatment (P<0.05; Figure 5B, 5C).
Discussion
PC12 cells, which are derived from rat phaeochromocytoma, contain a more homogeneous population, develop a faithful neuronal phenotype, and are available in large amounts for biomedical study. Thus, PC12 cells have been widely used in both neurobiological and neurotoxicological studies[25]. In the present study, cell viability was detected in the PC12 cells by MTT assay. As expected, there were significantly less viable cells in the ethanol-treated cells as compared with the controls. However, incubation with Tan IIA before adding ethanol increased cell viability and raised the survival rate.
The LDH enzyme release and the formation of ROS confirmed the results obtained from the MTT assay. A breakdown in the integrity of the plasma membrane results in the release of LDH into the extracellular space. LDH activity in the extracellular fluid of cells exposed to ethanol was greater than that observed in cells exposed to ethanol and Tan IIA. It has been shown that chronic ethanol treatment can also cause the formation of ROS and nitrogen species and the activation of the mitochondrial permeability transition[26]. In the present study, the PC12 cells exposed to high concentrations of ethanol showed an increased formation of ROS. However, incubation with Tan IIA reduced the formation of ROS. All corresponded to the decrease in viability as assessed by MTT assay. These results favor the view that oxidative damage is mainly responsible for the cytotoxicity of ethanol and that Tan IIA can reverse the effects.
It has been reported that ethanol can induce cell apoptosis and necrosis or both[27–29], but whether Tan IIA can attenuate the process is still unknown. Because programmed cell death follows an orchestrated sequence of events, temporal markers of apoptosis occur in stages. Early apoptosis coincides with subtle membrane disruption leading to a change of topology in the bilayer so that phosphatidylserine that mostly reside in the cytoplasmic leaflet can also be detected in the outer leaflet. PI can permeate necrotic cell membrane, and then bind nucleic acids. Thus, in the present study, annexin-PI analysis, direct assay of apoptotic death, was used to detect the effects of Tan II A on cell apoptosis and necrosis induced by ethanol. Figure 4 shows a bivariate annexin V/PI analysis of PC12 cells. From this, it is clear that ethanol induced massive apoptosis and a large part of the cells were at late apoptotic stage. Although Tan IIA alone did not induce any increase of apoptosis, which was essentially similar to the control cells, pretreatment with Tan IIA was effective in abrogating the apoptosis induced by ethanol. This protective effect of Tan IIA against ethanol-induced apoptosis might be because of its radical scavenging capacity. This view coincided with our previous findings that tanshinone attenuated aminoglycoside-induced free radical formation both in vitro and in vivo[14].
To further study the mechanism of Tan IIA on the apoptosis of PC12 cells induced by ethanol, we detected the protein expression of p53 by immuno-fluorescence and flow cytometry. When cells are injured, p53 is one of the major mediators of apoptosis, implicated in tumors of the central nervous system, neurological disorders, aging, as well as the death of development neurons in the cerebellum, hippocampus, and cerebral cortex. Moreover, upregulation of p53 activates mediators of apoptosis, such as caspase-3 and caspase-9 and is pro-apoptotic for neurons. In addition, the apoptosis in PC12 cells is mainly controlled by p53 and the Bcl-2 family[30]. In the present study, the results of immuno-fluorescence and flow cytometry show that Tan IIA can reduce the p53 protein expression, which coincides with the decreased apoptosis in the PC12 cells treated with Tan IIA. Thus, our results indicate that p53 might mediate ethanol-induced PC12 cells apoptosis.
In summary, Tan IIA can protect the neurotoxicity induced by ethanol by increasing the cell viability and decreasing the formation of ROS. It can also reduce cell apoptosis which may result from the inhibition of the pro-apoptosis p53 expression. All these results suggest that Tan IIA might serve as a potential therapeutic drug for neurological disorders induced by ethanol.
Acknowledgements
We are grateful to Prof Zhi-hui LIANG, Hui-fen ZHU, and the flow cytometry workgroup for their technical advice.
References
- Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, Fontaine M, et al. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc Natl Acad Sci USA 2002;99:6398-403.
- Light KE, Belcher SM, Pierce DR. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience 2002;114:327-37.
- Moulder KL, Fu T, Melbostad H, Cormier RJ, Isenberg KE, Zorumski CF, et al. Ethanol-induced apoptosis in the developing visual system during synaptogenesis. Invest Ophthalmol Vis Sci 2003;44:2809-17.
- Watts LT, Rathinam ML, Schenker S, Henderson GI. Astrocytes protect neurons from ethanol-induced oxidative stress and apoptotic death. J Neurosci Res 2005;80:655-66.
- Dikranian K, Qin YQ, Labruyere J, Nemmers B, Olney JW. Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Brain Res Dev Brain Res 2005;155:1-13.
- Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegen-eration in the developing brain. Brain Pathol 2002;12:488-98.
- Gohlke JM, Griffith WC, Bartell SM, Lewandowski TA, Faustman EM. A computational model for neocortical neuronogenesis predicts ethanol-induced neocortical neuron number deficits. Dev Neurosci 2002;24:467-77.
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res 2004;28:941-8.
- Han JY, Joo Y, Kim YS, Lee YK, Kim HJ, Cho GJ, et al. Ethanol induces cell death by activating caspase-3 in the rat cerebral cortex. Mol Cells 2005;20:189-95.
- Maas JW Jr, Indacochea RA, Muglia LM, Tran TT, Vogt SK, West T, et al. Calcium-stimulated adenylyl cydases modulate ethanol-induced neurodegeneration in the neonatal brain. J Neurosci 2005;25:2376-85.
- Tizabi Y, Manaye KF, Taylor RE. Nicotine blocks ethanol-induced apoptosis in primary cultures of rat cerebral cortical and cerebellar granule cells. Neurotox Res 2005;7:319-22.
- Young C, Klocke BJ, Tenkova T, Choi J, Labruyere J, Qin YQ, et al. Ethanol-induced neuronal apoptosis in vivo requires BAX in the developing mouse brain. Cell Death Differ 2003;10:1148-55.
- Liao SL, Chen WY, Raung SL, Chen CJ. Ethanol attenuates ischemic and hypoxic injury in rat brain and cultured neurons. Neuroreport 2003;14:2089-94.
- Li LX, Dai JP, Ru LQ, Yin GF, Zhao B. Effects of tanshinone on neuropathological changes induced by amyloid beta-peptide(1-40) injection in rat hippocampus. Acta Pharmacol Sin 2004;25:861-8.
- Lam BY, Lo AC, Sun X, Luo HW, Chung SK, Sucher NJ. Neuro-protective effects of tanshinones in transient focal cerebral ischemia in mice. Phytomedicine 2003;10:286-91.
- Wang AM, Sha SH, Lesniak W, Schacht J. Tanshinone (Salviae miltiorrhizae extract) preparations attenuate aminoglycoside-induced free radical formation in vitro and cytotoxicity in vivo. Antimicrob Agents Chemother 2003;47:1836-41.
- Mostallino MC, Mascia MP, Pisu MG, Busonero F, Talani G, Giovanni B. Inhibition by miltirone of up-regulation of GABAA receptor α4 subunit mRNA by ethanol withdrawal in hippocampal neurons. Eur J Pharmacol 2004;494:83-90.
- Ji ZN, Liu GQ. Inhibition of serum deprivation-induced PC12 cell apoptosis by tanshinone II A. Acta Pharmacol Sin 2001;22:459-62.
- Kang K, Oh YK, Choue R, Kang SJ. Scutellariae radix extracts suppress ethanol-induced caspase-11 expression and cell death in N(2)a cells. Brain Res Mol Brain Res 2005;142:139-45.
- Hirata H, Machado LS, Okuno CS, Brasolin A, Lopes GS, Smaili SS. Apoptotic effect of ethanol is potentiated by caffeine-induced calcium release in rat astrocytes. Neurosci Lett 2006;393:136-40.
- Tizabi Y, Manaye KF, Taylor RE. Nicotine blocks ethanol-induced apoptosis in primary cultures of rat cerebral cortical and cerebellar granule cells. Neurotox Res 2005;7:319-22.
- Broniszewska-Ardelt B, Wroblewski JT. The activity of phospho-fructokinase, pyruvate kinase and lactate dehydrogenase in guinea pig and rat brains in normal and hypoxic conditions. Bull Acad Pol Sci Biol 1975;23:505-12.
- Iannone F, De Bari C, Scioscia C, Patella V, Lapadula G. Increased Bcl-2/p53 ratio in human osteoarthritic cartilage: a possible role in regulation of chondrocyte metabolism. Ann Rheum Dis 2005;64:217-21.
- Quinones A, Rainov NG. Identification of genotoxic stress in human cells by fluorescent monitoring of p53 expression. Mutat Res 2001;494:73-85.
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 1976;73:2424-8.
- Pastrino JG, Hoek JH. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology 2000;31:1141-52.
- Moulder KL, Fu T, Melbostad H, Cormier RJ, Isenberg KE, Zorumski CF, et al. Ethanol-induced death of postnatal hippocampal neurons. Neurobiol Dis 2002;10:396-409.
- Ikegami Y, Goodenough S, Inoue Y, Dodd PR, Wilce PA, Matsumoto I. Increased TUNEL positive cells in human alcoholic brains. Neurosci Lett 2003;349:201-5.
- Nowoslawski L, Klocke BJ, Roth KA. Molecular regulation of acute ethanol-induced neuron apoptosis. J Neuropathol Exp Neurol 2005;64:490-7.
- Vaghefi H, Hughes AL, Neet KE. Nerve growth factor withdrawal-mediated apoptosis in naive and differentiated PC12 cells through p53/caspase-3-dependent and -independent pathways. J Biol Chem 2004;279:15604-14.
