Chemokine RANTES is upregulated in monocytes from patients with hyperhomocysteinemia1
Introduction
Elevated plasma homocysteine (Hcy) concentration is an independent risk factor for cardiovascular disease[1,2]. Although the exact mechanism by which hyperhomo-cysteinemia (HHcy) induces cardiovascular disease is still uncertain, recent evidence suggests that HHcy may induce the process of atherogenesis by initiating inflammation. Hcy may affect inflammatory cells, such as mononuclear leukocytes, monocytes, and T cells, as well as multiple cytokines, to alter the inflammatory status of endothelial cells, smooth muscle cells (SMC) and intimal macrophages during the formation of lesions[3,4]. Hcy induces the expression and secretion of monocyte chemoattractant protein -1 (MCP-1) and interleukin-8 (IL-8) in human aortic endothelial cells, SMC lines, and monocyte cells[5–8]. Furthermore, we have shown that Hcy not only promotes the secretion of MCP-1 and IL-8 but also enhances their gene expression in primary cultured human monocytes[7]. Holven et al[9] also reported that the plasma level of the chemokine epithelial neutrophil-activating peptide-78 (ENA-78) and growth-regulated oncogene (GROα) was elevated in patients with HHcy. This evidence suggests a role of pro-inflammatory chemokines in HHcy-initiated atherogenesis.
RANTES (regulated upon activation, normal T cells expressed and secreted) is a CC chemokine, normally derived by T lymphocytes. It is also produced by mono/macrophages. It plays an important role in the inflammatory process. It has been found to play an important role in autoimmune dis-orders such as asthma or systemic lupus erythematosus and is nearly as potent a chemoattractant for monocytes as MCP-1[10,11]. To date, no evidence exists on the relation between HHcy and RANTES. In the present study, we determined the plasma level of RANTES and the responsiveness of monocytes to low-dose lipopolysaccharide (LPS)-induced RANTES secretion in patients with HHcy and elucidated the direct effect of Hcy on RANTES mRNA level in human monocytes.
Materials and methods
Subjects Two groups of subjects were studied: 40 patients with HHcy (plasma Hcy level more than 15 µmol/L) and 38 control patients with normal blood Hcy level (NHcy). All subjects had no infectious diseases, malignancies or immunologic or hematological diseases; were not being treated with anti-inflammatory drugs other than low-dose aspirin; did not use any vitamins including folic acid during the study period; had no depressed cardiac function (ejection fraction <40%); were younger than 75 years; had no renal failure; and had not had acute myocardial infarction in the previous 3 months.
Venous blood samples were obtained from fasting subjects to assay the chemokines from plasma and isolated monocytes stimulated by low-dose LPS. All subjects gave their written, informed consent. This study was approved by the Ethics Committee of the Health Science Center, Peking University.
Measurement of RANTES The supernatant of cultured monocytes and plasma stored at -70 °C were used to measure the chemokines. The concentration of RANTES was determined by ELISA according to the manufacturer’s protocols (R&D Systems, Minneapolis, MN, USA).
Measurement of plasma level of Hcy and folate A plasmid-encoding human placental AdoHcy hydrolase (pPROK-1) kindly provided by the University of Kansas (USA) was overexpressed in E coli JM109 cells transformed with pPROK-1 and purified as described in a previous study[12]. Total Hcy level in plasma was determined with the use of an assay based on the conversion of Hcy to S-adenosyl-homo-cysteine (SAH) in the presence of adenosine and SAH hydrolase as described from our previous study[8]. Briefly, plasma samples from fasting subjects were collected. Protein-bound circulating Hcy was reduced to free Hcy with the use of dithioerythreitol as a reductant. The free Hcy was then converted to SAH by using SAH hydrolase and excess adenosine. Samples were quantified by HPLC. The range of measurement was 1–100 µmol/L, with a sensitivity of less than 0.5 µmol/L. The within-assay and between-assay coefficients of variation were 3.5% and 4.9%, respectively.
Plasma folate was measured by radioisotope dilution assay with use of a kit from Nanjing Jiangcheng Bioengineering Institute (Nanjing, China).
Responsiveness of human monocytes to LPS Blood of HHcy and NHcy patients was drawn into heparinized syringes. The whole blood was separated into peripheral blood mononuclear cells and neutrophils by use of the density gradient from Nycoprep 1.077 (Life Technologies, Grand Island, NY, USA). Monocytes were then isolated from the peripheral blood mononuclear cells by their adherence to a serum-coated culture flask for 2 h. Adherent cells were then detached and resuspended in RPMI-1640 medium containing 5% autologous plasma. Freshly isolated monocytes (5×105) were incubated at 37 ºC and treated with or without LPS (final concentration 0.01–0.1 µg/mL) for 24 h. The cell-free supernatant was harvested and stored at -70 ºC for chemokine analysis. Cellular viability was determined with Trypan blue exclusion. Only cell preparations with 95% viability or greater were used.
RNase protection assays Total RNA was isolated from healthy human monocytes with Trizol Reagent (Life Technologies). Assays involved use of a nuclease protection assay kit (Riboquant, Pharmingen, San Diego, CA, USA) as used in our previous published report[7]. In summary, the isolated RNA (2 mg) was hybridized with 32P end-labeled RANTES oligonucleotide probes overnight at 30 °C, followed by nuclease digestion. A GAPDH and L-32 rRNA oligonucleotide probe was used as an internal control. After digestion, the protected fragments were resolved on a denatured 12% polyacrylamide gel containing 8 mol/L urea, then transferred to filter paper, which was later exposed to X-ray film. The bands corresponding to RANTES, L-32 or GAPDH mRNA were analyzed by use of a gel documentation system (Cold-Spring Eletro Doc and Analyst Edas 290, New York, USA).
Statistical analysis Because chemokine and Hcy values do not follow a normal distribution in patients, comparisons between groups involved the use of the Mann-Whitney test. Values are expressed as median, 25th and 75th percentile, and 10th and 90th percentile. Patients’ age, body-mass index and plasma level of cholesterol, triglycerides and creatinine were analyzed with use of the Student’s t-test and expressed as mean±SD. Proportions were analyzed by use of the Chi-squared test. P<0.05 (two-tailed) was considered statistically significant.
Results
Clinical characteristics of the study participants The NHcy control and HHcy groups did not differ significantly with respect to age, sex, and body-mass index. The prevalence of smoking, coronary artery disease, hypertension and diabetes, as well as base-line demographic characteristics and laboratory values, were also similar in the two groups (Table 1).
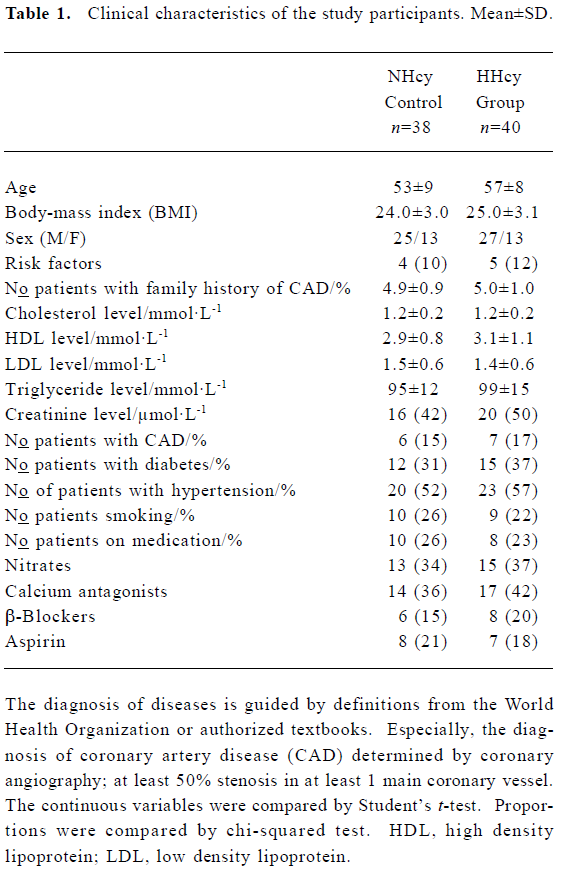
Full table
Plasma level of Hcy, folate, and RANTES The Hcy level was significantly higher in HHcy subjects than that in NHcy controls, while the folate level was lower in HHcy subjects than that in NHcy controls. In the HHcy group, the plasma level of RANTES was significantly elevated (median 5.3 ng/mL) compared with NHcy controls (3.5 ng/mL) (Table 2).
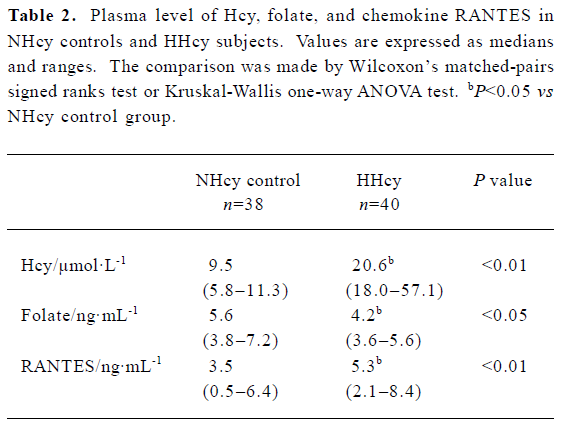
Full table
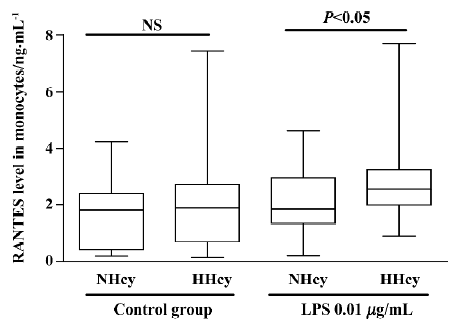
Chemokine secretion from isolated monocytes in response to LPS To test whether monocytes isolated from patients with HHcy exhibited enhanced inflammatory response of RANTES production, monocytes were incubated with low-dose LPS (0.01 µg/mL) for 24 h. As shown in Figure 1, RANTES production was not significantly increased in HHcy patients without LPS stimulation, but LPS treatment (0.01 µg/mL) significantly increased the level of RANTES secretion of monocytes from HHcy patients compared with that of NHcy controls. Thus, monocytes from patients with HHcy may show an enhanced RANTES secretion response in a pro-inflammatory condition.
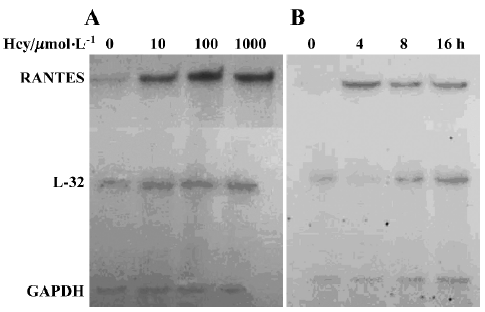
Effect of Hcy on RANTES mRNA expression To determine whether Hcy modulates the expression of RANTES mRNA, total RNA was isolated from normal human monocytes treated with Hcy at various times and at different concentrations. As shown in Figure 2, RNase protection assays revealed that the expression of RANTES mRNA was significantly enhanced after Hcy treatment. Cultured monocytes incubated with Hcy (10–1000 µmol/L) for 8 h showed significantly increased RANTES expression as compared with untreated cells (Figure 2A), beginning as low as Hcy 10 µmol/L. The increased level of RANTES mRNA after incubation with Hcy 100 μmol/L peaked at 4–8 h after incubation (Figure 2B).
Discussion
The results from the present study demonstrated that the plasma RANTES level was elevated in HHcy individuals and RANTES secretion from monocytes was increased. We also provided evidence that Hcy directly induced RANTES mRNA synthesis from isolated human monocytes.
Atherosclerosis is a chronic inflammatory disease characterized by the recruitment of monocytes and lymphocytes to the artery wall. Increasing evidence supports the involvement of inflammation in the early phases of atherogenesis. Recruitment of leukocytes within the vascular wall is an essential process in the development of this common disease, which is mainly regulated by adhesion to molecules and chemokines[3,4]. In the classic inflammatory response, adhesion is followed by transmigration of the leukocytes through the endothelial layer into the intima. This process is also mediated by chemotactic factors.
A number of studies have determined the important role of chemokines such as MCP-1 and IL-8 in the initial stages of atherosclerotic plaque formation[5,6]. RANTES, a CC chemokine, has been found to play an important role in autoimmune injury to several tissues. RANTES is generated by circulatory lymphocytes and some kinds of tissue cell monocytes. RANTES is nearly as potent a chemoattractant for monocytes as MCP-1. RANTES induces leukocyte transendothelial migration, implicated in the initial stages of the inflammatory part of the atherosclerotic process[13]. Furthermore, evidence implies the important role RANTES plays in atherosclerosis. Simeoni et al[14] reported that a mutant RANTES genotype was associated with the increased coronary heart disease death rate, independent of conventional risk factors. A causal role for RANTES in atherosclerosis was also shown by a protective effect of the blockage of RANTES receptors with the CC chemokine antagonist Met-RANTES in an ApoE-deficient hypercholesterolemic mouse model[15]. These data suggest that the alteration of the level of the chemokine RANTES may affect the progress of atherogenesis through the inflammatory pathway.
Hyperhomocysteinemia is found in 30% of patients with premature atherosclerosis of carotid and peripheral arteries. Elevated plasma Hcy levels have been implicated as an independent risk factor for coronary heart disease[16,17]. Therefore, intensive studies have focused on whether HHcy is the cause or merely a marker for cardiovascular disease. Hcy significantly enhances MCP-1 and IL-8 levels in healthy human monocytes, which can increase leukocyte chemotaxis[7]. However, compared with studies of MCP-1 and IL-8, little study has focused on RANTES secretion in HHcy. Economou et al[17] reported the negative association of circulating Hcy and RANTES in prepubescent lean children. Until now, no direct evidence of the relation between HHcy and RANTES in human monocytes exists.
In the present study, we found that both the plasma level of RANTES and low-dose LPS-induced RANTES production in isolated monocytes from patients with HHcy were significantly elevated. Hcy not only increased the RANTES level in HHcy patients but also increased RANTES gene expression in primary human monocytes. Thus, our results support our hypothesis that HHcy may play an important role in the pathogenesis of atherosclerosis through a mechanism involving an increase in RANTES secretion in human monocytes.
Our recent published data show that folate acid treatment in HHcy patients can reverse the hyper-responsiveness of MCP-1 and IL-8 secretion from monocytes[8]. On the basis of the current results, further study should focus on whether the intervention of HHcy influences monocyte-derived RANTES secretion and also the exact mechanism underlying the Hcy-upregulated RANTES production.
References
- Wald NJ, Watt HC, Law MR, Weir DG, Mcpartlin J, Scott JM. Homocysteine and ischemic heart disease: results of a prospective study with implications regarding prevention. Arch Int Med 1998;158:862-7.
- Tsai JC, Perrella MA, Yoshizumi M, Hsieh CM, Haber E, Schlegel R, et al. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci USA 1994;91:6369-73.
- Sheikine Y, Hansson GK. Chemokines and atherosclerosis. Ann Med 2004;36:98-118.
- Bursill CA, Channon KM, Greaves DR. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr Opin Lipidol 2004;15:145-9.
- Poddar R, Sivasubramanian N, Dibello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation 2001;103:2717-23.
- van Aken BE, Jansen J, van Deventer SJ, Reitsma PH. Elevated levels of homocysteine increase IL-6 production in monocytic Mono Mac 6 cells. Blood Coagul Fibrinolysis 2000;11:159-64.
- Zeng X, Dai J, Remick DG, Wang X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Circ Res 2003;93:311-20.
- Wang G, Dai J, Mao J, Zeng X, Yang X, Wang X. Folic acid reverses hyper-responsiveness of LPS-induced chemokine secretion from monocytes in patients with hyperhomocysteinemia. Atherosclerosis 2005;179:395-402.
- Holven KB, Aukrust P, Holm T, Ose L, Nenseter MS. Folic acid treatment reduces chemokine release from peripheral blood mononuclear cells in hyperhomocysteinemic subjects. Arterioscler Thromb Vasc Biol 2002;22:699-703.
- Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 1990;347:669-71.
- Rollins BJ. Chemokines. Blood 1997;90:909-28.
- Chen J, Liu Q, Yang X, Wang K. Copper ions inactivate S-adenosyl-L-homocysteine hydrolase. Chin Sci Bull 2002;47:1176-9.
- Simeoni E, Winkelmann BR, Hoffmann MM, Fleury S, Ruiz J, Kappenberger L, et al. Association of RANTES G-403A gene polymorphism with increased risk of coronary arteriosclerosis. Eur Heart J 2004;25:1378-81.
- Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res 2004;94:253-61.
- Malinow MR, Nieto FJ, Szklo M, Chambless LE, Bond G. Carotid artery intimal-medial wall thickening and plasma homocyst(e)ine in asymptomatic adults. The Atherosclerosis Risk in Communities Study. Circulation 1993;87:1107-13.
- Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl Med 1999;324:1149-55.
- Economou EV, Malamitsi-Puchner AV, Pitsavos CP, Kouskouni EE, Magaziotou-Elefsinioti I, Damianaki-Uranou D, et al. Negative association between circulating total homocysteine and proinflammatory chemokines MCP-1 and RANTES in prepubertal lean, but not in obese, children. J Cardiovasc Pharmacol 2004;44:310-5.
