Synergistic effects of atenolol and amlodipine for lowering and stabilizing blood pressure in 2K1C renovascular hypertensive rats1
Introduction
Blood pressure (BP) is not constant, and can vary spontaneously. This variation is defined as blood pressure variability (BPV). BPV is increased in hypertensive humans and animals[1-3]. Furthermore, BPV is positively related to the severity of organ damage in hypertensive humans and rats[4–6]. In other words, increased BPV can produce organ damage. Therefore, it has been proposed that antihypertensive drugs with a BP-stabilizing effect would act to protect organs in the treatment of hypertension. We have demonstrated that long-term treatment with ketanserin, candesartan, nitrendipine, a combination of nitrendipine and atenolol, and a hydrochlorothiazide mixture not only decrease BP, but also decrease BPV, and have obvious effects on organ protec-tion in spontaneously hypertensive rats as well. Importantly, organ protection was attributed to the decrease in BPV[7–10].
Clinically, combination therapy against hypertension using 2 or more drugs from different classes can produce better drug efficacy[11]. Furthermore, the use of such synergistic therapy is also recommended for the initial treatment of hypertension[12]. Both β-blockers and dihydropyridine calcium antagonists are widely used in the treatment of hypertension. Atenolol and amlodipine are examples of long-acting drugs from these 2 classes. We hypothesized that these 2 drugs combined would produce a synergistic effect in the treatment of hypertension. Therefore, this study was designed to investigate the synergistic effects of atenolol and amlodipine on both lowing and stabilizing BP in 2K1C renovascular hypertensive rats (RVHR).
Materials and methods
Drugs and drug administration Amlodipine (Nanjing Pharmaceutical Co, Nanjing, China), atenolol (Shanghai Second Pharmaceutical Co, Shanghai, China) and a combination of these 2 drugs were dissolved in 0.8% carboxymethylcellulose sodium (CMC). The doses used were as follows: atenolol 10.0 mg/kg, amlodipine 1.0 mg/kg and 5.0+0.5 mg/kg, 10.0+1.0 mg/kg, and 20.0+2.0 mg/kg combinations of the 2 drugs. Five groups of rats received antihypertensive drugs and one group of rats received 0.8% CMC as control. Drugs were administered by a catheter in a gastric fistula implanted 3 days before treatment.
Animals and RVHR preparation Male Sprague–Dawley rats (160–180 g), purchased from the Sino-British SIPPR/BK Lab Animal Ltd (Shanghai, China), were anesthetized with a combination of ketamine (40 mg/kg) and diazepam (6 mg/kg). The right renal artery of each animal was isolated through a flank incision as described previously[13], and a silver clip (0.2 mm internal gap) was placed on the renal artery. Five weeks after placement of the clip, the systolic blood pressure (SBP) of rats was measured by using the tail-cuff method (CB10; Alcott Biotech Co, Shanghai, China). In total, 48 RVHR whose SBP was greater than 160 mmHg were used in this study. Rats were kept in a controlled temperature (23 °C–25 °C) and lighting (light 08:00–20:00, dark 20:00–08:00) environment, and had free access to food and tap water. All the animals used in this work received humane care in compliance with institutional animal care guidelines.
BP and BPV measurement SBP, diastolic blood pressure (DBP) and heart period (HP) were continuously recorded using a previously described technique[10]. Briefly, rats were anesthetized by injection (ip) with a combination of ketamine (40 mg/kg) and diazepam (6 mg/kg). A floating polyethylene catheter was inserted into the lower abdominal aorta via the left femoral artery for BP measurement, and another catheter was placed into the stomach via a mid-abdominal incision for drug administration. The catheters were exteriorized through the interscapular skin. After a 2-d recovery period, the animals were placed for BP recording in individual cylindrical cages containing food and water. The aortic catheter was connected to a BP transducer via a rotating swivel that allowed the animals to move freely in the cage. After approximately 14-h habituation, at 9:00 o’clock the BP signal was begun to be digitized by a microcomputer. One hour later, at 10:00 o’clock the drug was given via the catheter in the gastric fistula. SBP, DBP, and HP values were recorded beat-to-beat for 25 h, up to 10:00 o’clock on the second day. The mean values of these parameters during a designated period were calculated and served as SBP, DBP and HP values. The standard deviation of all values obtained over 24 h was denoted as the quantitative parameter of variability; that is, SBP variability (SBPV), DBP variability (DBPV), and HP variability (HPV) for each rat.
Probability sum test To determine whether the drugs were acting synergistically, we used the probability sum test. This test comes from classic probability analysis and is useful for evaluating the synergistic interactions between 2 drugs (q test)[10,14,15].
In the present work, we used the following criteria. Compared with the mean values from control rats, treated rats with a decrease in BP (SBP or DBP) >20 mmHg were defined as responders and rats with a decrease in BP≤20 mmHg were defined as non-responders. For BPV (SBPV or DBPV), the criterion was 2 mmHg. The formula used is as follows: q=PA+B/(PA+PB–PA×PB). Here, A and B indicate drug A and drug B; P (probability) is the percentage of responders in each group. PA+B is the real percentage of responders and (PA+PB–PA×PB) is the expected response rate. PA+PB is the sum of the probabilities when drug A and drug B are used alone. PA×PB is the probability of rats responding to both drugs when they were used alone. When q<0.85, the combination is antagonistic, when q>1.15, the combination is synergistic, and when q is between 0.85 and 1.15, the combination is additive.
Statistical analysis Data were expressed as mean±SEM. Comparisons between values obtained in the same group before and after drug administration were made using the paired t-test. Comparisons among groups were carried out using ANOVA followed by Duncan’s test. P<0.05 was considered statistically significant.
Results
Effects of atenolol and amlodipine on BP and HP at 24 h after treatment The effects of atenolol and amlodipine alone and in combination on BP and HP at 24 h after administration in 2K1C RVHR are shown in Table 1. The mean values of these variables 1 h before administration serve as the control values and are defined as “before”; the mean values at 24 h after administration are defined as “after”. We found that SBP was significantly decreased in all 3 groups treated with both atenolol and amlodipine when compared with “before”. A significantly lower DBP was also found in 2 groups of rats treated with both atenolol and amlodipine (10.0+1.0 mg/kg and 20.0+2.0 mg/kg) when compared with “before”. Relative to “before”, the mean HP value at 24 h after administration was higher only in rats treated with atenolol (10.0 mg/kg) alone. Of the 3 groups treated with both atenolol and amlodipine, we found that both the intermediate and the high doses reduced SBP at 24 h after administration more effectively than the low dose.
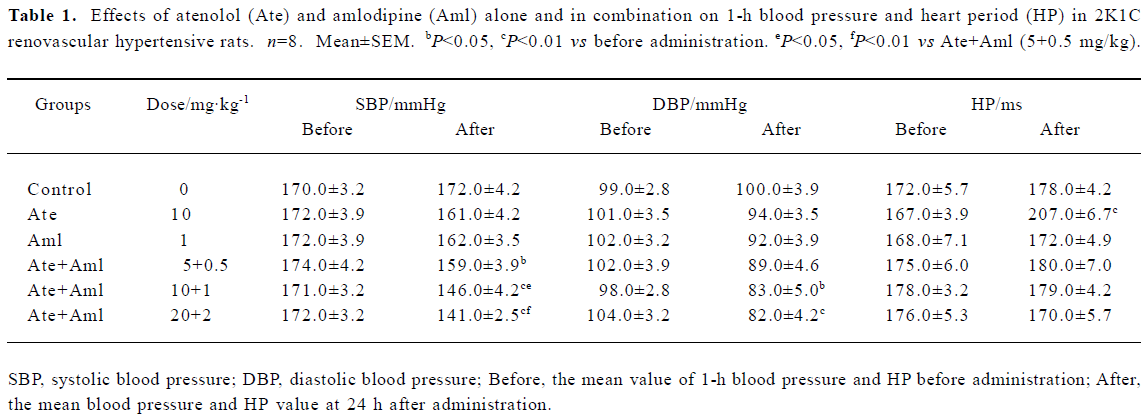
Full table
Effects of atenolol and amlodipine on BP and HP for the 24-h period after administration We found that the average SBP in control rats during the 24 h after administration was 171.0±3.5 mmHg. The average SBP over this time was significantly lower in all 5 groups treated with either atenolol, or amlodipine, or both when compared with the control group (Figure 1). The minimal decrease (-13.0 mmHg) was found in rats treated with amlodipine alone and the maximal decrease (-39.0 mmHg) was found in rats treated with both atenolol and amlodipine at a high dose (20.0+2.0 mg/kg). Compared with control, the decrease in average DBP in the experimental groups during the 24 h after administration was also significant but not so profound. The mean value of HP during this time was only increased in the group treated with atenolol (10.0 mg/kg) alone. Of the 3 groups treated with both atenolol and amlodipine, both the intermediate and the high doses reduced SBP more obviously than the low dose (Figure 1).
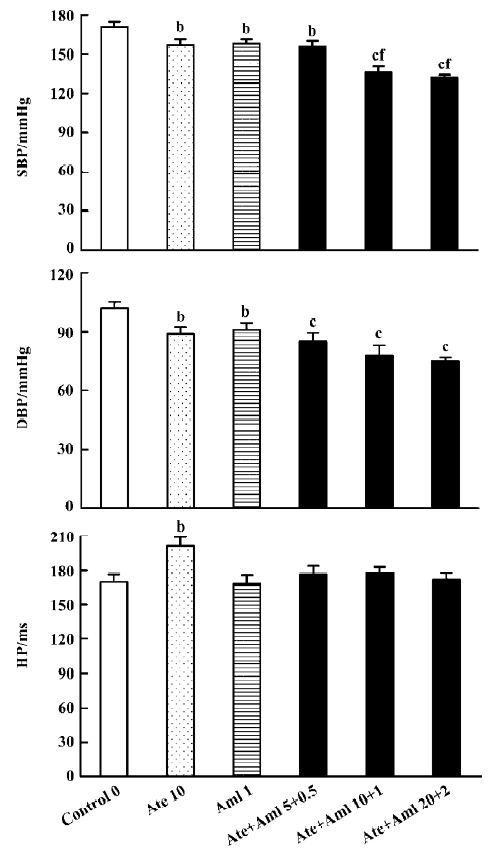
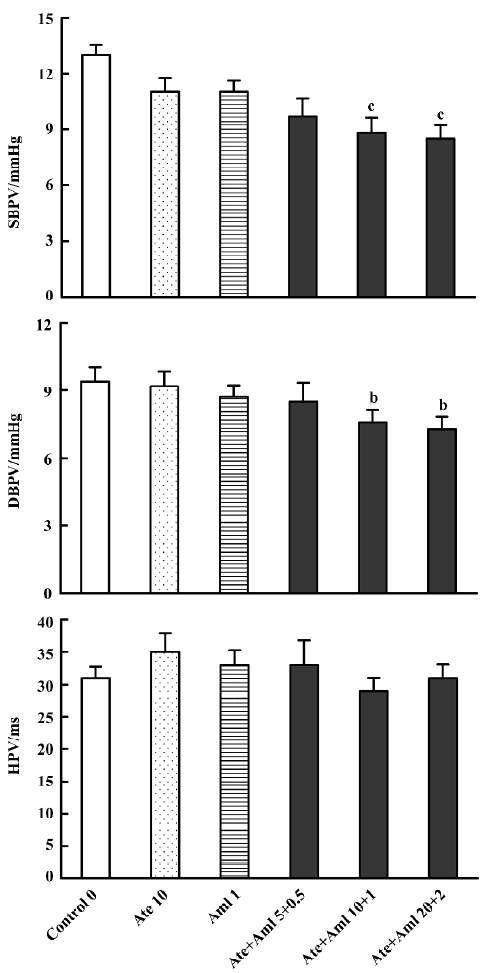
Effects of atenolol and amlodipine on BPV and HPV for the 24-h period after administration Compared with the control group (13.00±0.53 mmHg for SBPV and 9.40±0.64 mmHg for DBPV), a significant decrease in BPV was found in groups treated with both atenolol and amlodipine at an intermediate (8.80±0.85 mmHg for SBPV and 7.60±0.53 mmHg for DBPV) and high dose (8.50±0.71 mmHg for SBPV and 7.30±0.53 mmHg for DBPV) (Figure 2).
Synergistic interaction of atenolol and amlodipine on BP and BPV for the 24-h period after administration Based on the results presented in Figure 1, the effectiveness of the decrease in BP was calculated for rats individually. Rats with a decrease in BP >20 mmHg (relative to controls) were defined as responders and those with a decrease in BP ≤20 mmHg as non-responders. The results of probability testing are presented in Table 2. We arrived at q values of 2.29 for SBP and 1.45 for DBP for the combination of both atenolol and amlodipine (10.0+1.0 mg/kg). Compared with the mean values for control rats, rats with a decrease in SBPV or DBPV >2 mmHg were defined as responders. According to this criterion, the q values were 1.41 for SBPV and 1.60 for DBPV for the combination of both atenolol and amlodipine (10.0+1.0 mg/kg).
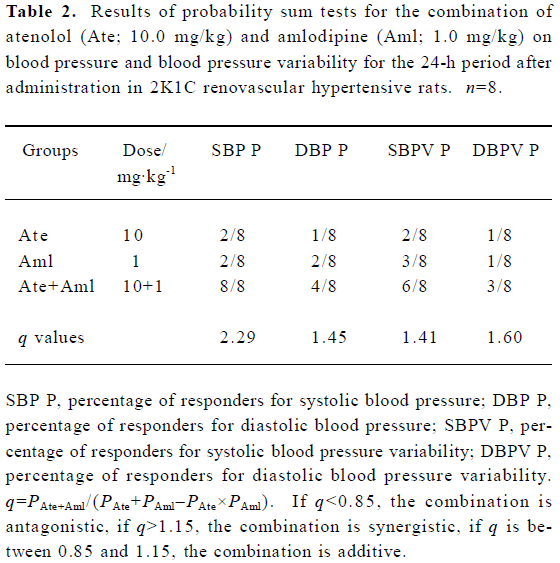
Full table
Discussion
The purpose of using fixed-dose combinations of 2 different kinds of antihypertensive drugs in the treatment of hypertension is to obtain increased BP control and to enhance compliance by using a single tablet that is taken once or twice daily[16]. Furthermore, by combining 2 different agents at lower doses, the clinical and metabolic side effects that would be produced by either drug at higher doses can be minimized[17]. Therefore, fixed-dose combinations of antihypertensive drugs could potentially increase BP control, simplify dosage regimens, improve compliance, decrease dose-dependent side effects, and reduce costs as the first-line treatment for hypertension[18]. These advantages make combination antihypertensive therapy the recommended initial treatment, particularly in patients with end-organ damage (EOD) or more severe initial hypertension[19,20]. However, high BP is not a unique factor determining hypertensive EOD. Parati et al found that for patients with similar mean hypertension levels for the 24 h after treatment, those whose BPV levels were lower had less severe organ damage than those with higher BPV levels[4]. Our previous study found that in 60-week-old spontaneously hypertensive rats, the severity of organ damage was positively related to BP (r=0.31–0.32, n=50, P<0.05) and BPV levels (r=0.63–0.65, n=50, P<0.01), and BPV was positively related to the extent of organ damage, so BPV might be a new determinant of EOD[6,21,22].
Therefore, it seems very important to emphasize the role of BPV in antihypertensive therapy. However, it is not clear how BPV can be controlled in the treatment of hypertension. We have previously proposed 2 ways to reduce BPV in antihypertensive therapy[6]: (1) find antihypertensive drugs that decrease BPV even at a dose that does not affect BP, for example ketanserin; and (2) treat patients with long-acting antihypertensive drugs, for example candesartan or amlo-dipine. In the present work, we investigated a third way to control BPV in the treatment of hypertension, that is, combination therapy.
Both β-blockers and dihydropyridine calcium antagonists are widely used in antihypertensive therapy. The combination of a β-blocker and a dihydropyridine calcium antagonist is a logical choice[23], and is effective for treating hypertensive patients with chronic renal insufficiency and left ventricular hypertrophy[24]. Theoretically, calcium antagonists are vasodilators and tend to increase plasma renin, therefore combining them with β-blockers is a good idea[25]. A combination of these compounds can also neutralize the side effects of both, for example the initial heart rate increases induced by dihydropyridine calcium antagonists, and the rise in peripheral resistance elicited by some β-blockers[26].
In terms of the synergistic effects of atenolol (10.0 mg/kg) and amlodipine (1.0 mg/kg) on BP reduction, the findings of the present work are: (1) the combination of atenolol and amlodipine significantly decreases BP 24 h after administration, whereas neither drug alone had an obvious influence; (2) both atenolol or amlodipine alone reduced the average BP for the 24 h period after administration by less than 15 mmHg, but in combination they decreased SBP by up to 35 mmHg and DBP by approximately 25 mmHg. The q values for SBP and DBP for the 24 h period after administration were 2.29 and 1.45, respectively, which are higher than 1.15, the threshold value for synergistic effects. Concerning the synergism of combined atenolol and amlodipine on the reduction in BPV for the 24 h period after administration, we found that BPV was not influenced by treatment with atenolol or amlodipine alone, but was markedly reduced when they were used in combination. The q values for SBPV and DBPV for the 24 h period after administration were 1.41 and 1.60, respectively. These findings demonstrate that the combination of atenolol and amlodipine has a significant synergistic effect on lowing BP and stabilizing BPV in RVHR.
Our previous studies have demonstrated that long-term (4 months) treatment with a hydrochlorothiazide mixture, which consisted of 5 drugs at low doses, markedly reduced BP and BPV in spontaneously hypertensive rats[9]. Long-term (3 months) treatment with atenolol and nitrendipine had a significant protective effect on organs in spontaneously hypertensive rats[8].
We conclude that atenolol and amlodipine in combination have a synergistic effect in lowering and stabilizing BP in RVHR. Combination therapy is likely to be the optimal way to control BP and reduce BPV in the treatment of hypertension.
References
- Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res 1983;53:96-104.
- Su DF, Cerutti C, Barres C, Vincent M, Sassard J. Blood pressure and baroreflex sensitivity in conscious hypertensive rats of Lyon strain. Am J Physiol 1986;251:H1111-7.
- Miao CY, Shen FM, Su DF. Blood pressure variability is increased in genetic hypertension and L-NAME-induced hyperten-sion. Acta Pharmacol Sin 2001;22:137-40.
- Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 1987;5:93-8.
- Mancia G, Frattola A, Parati G, Santucciu C, Ulian L. Blood pressure variability and organ damage. J Cardiovasc Pharmacol 1994;24:S6-11.
- Su DF, Miao CY. Blood pressure variability and organ damage. Clin Exp Pharmacol Physiol 2001;28:709-15.
- Du WM, Miao CY, Liu JG, Shen FM, Yang XQ, Su DF. Effects of long-term treatment with ketanserin on blood pressure variability and end-organ damage in spontaneously hypertensive rats. J Cardiovasc Pharmacol 2003;41:233-9.
- Xie HH, Miao CY, Liu JG, Su DF. Importance of blood pressure variability in organ protection in spontaneously hypertensive rats treated with combination of nitrendipine and atenolol. Acta Pharmacol Sin 2002;23:1199-204.
- Lu ZA, Xie HH, Xu LP, Yin AF, Miao CY, Su DF. Restoration of arterial baroreflex function contributes to organ protection in spontaneously hypertensive rats treated with long-term hydrochlorothiazide mixture. Clin Exp Pharmacol Physiol 2003;30:49-54.
- Xie HH, Miao CY, Jiang YY, Su DF. Synergism of atenolol and nitrendipine on hemodynamic amelioration and organ protection in hypertensive rats. J Hypertens 2005;23:193-201.
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single-drug therapy for hypertension in men: a comparison of six antihypertensive agents with placebo: the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993;328:914-21.
- The sixth report of the Joint National Committee on preven-tion, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997;157:2413-46. [published erratum appears in Arch Intern Med 1998; 158: 573].
- Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin-converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension 1992;20:763-7.
- Jin ZJ. About the evaluation of drug combination. Acta Pharma-col Sin 2004;25:146-7.
- Su DF, Xu LP, Miao CY, Xie HH, Shen FM, Jiang YY. Two useful methods for evaluating antihypertensive drugs in conscious freely moving rats. Acta Pharmacol Sin 2004;25:148-51.
- Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med 1990;150:1881-4.
- Sica DA. Fixed-dose combination antihypertensive drugs. Do they have a role in rational therapy? Drugs 1994;48:16-24.
- Prisant LM. Fixed low-dose combination in first-line treatment of hypertension. J Hypertens 2002;20:S11-9.
- Moser M. Current recommendations for initial therapy in hyper-tension: are they still valid? Introduction. Am J Hypertens 1998;11:69S-72S.
- Moser M, Black HR. The role of combination therapy in the treatment of hypertension. Am J Hypertens 1998;11:73S-78S.
- Shan ZZ, Dai SM, Su DF. Relationship between baroreceptor reflex function and end-organ damage in spontaneously hypertensive rats. Am J Physiol 1999;277:H1200-6.
- Su DF, Miao CY. Reduction of blood pressure variability: a new strategy for treatment of arterial hypertension. Trends Pharma-col Sci 2005;26:388-90.
- Weir MR. The rationale for combination versus single-entity therapy in hypertension. Am J Hypertens 1998;11:163S-169S.
- Suzuki H, Moriwaki K, Kanno Y, Nakamoto H, Okada H, Chen XM. Comparison of the effects of an ACE inhibitor and alphabeta blocker on the progression of renal failure with left ventricular hypertrophy: preliminary report. Hypertens Res 2001;24:153-8.
- Waeber B, Detry JM, Dahlof B, Puig JG, Gundersen T, Hosie J, et al. Felodipine-metoprolol combination tablet: a valuable option to initiate antihypertensive therapy? Am J Hypertens 1999;12:915-20.
- Menard J. Critical assessment of combination therapy develop-ment. Blood Press 1993;1 Suppl:5-9.
