Changes of brain neuropeptide Y and its receptors in rats with flurazepam tolerance and dependence1
Introduction
Benzodiazepines (BDZ) have been widely used as anxiolytics, sedatives, hypnotics and anticonvulsants. BDZ produce therapeutic effects through the major fast inhibitory neurotransmitter receptors, GABAA receptors, in the central nervous system (CNS). BDZ positively modulate GABAA receptors by increasing their affinity for GABA and thus reduce the excitability of the post-synaptic neurons.
However, long-term treatment with BDZ often leads to drug tolerance, which means the therapeutic efficacy of the drug decreases after a period of continuous administration. Another consequence of chronic administration of BDZ is the dependence manifested by the aggravation of primary symptoms and/or appearance of abstinence syndrome, which includes anxiety, discomfort, diarrhea, loss of bodyweight and salivating when the drug is withdrawn abruptly. Tolerance and dependence are frequently encountered in the clinical use of BDZ as an antiepileptic medicine. The usefulness of BDZ is therefore limited, although most have primarily satisfactory antiepileptic efficacies.
Many studies have focused on the mechanisms of tolerance and dependence. Changes of binding capacity, gene expression and metabolism in neural receptors, including GABAA receptors[1], glutamatergic receptors[2] and peripheral BDZ receptors[3], were studied in the presence of tolerance and/or dependence. Other factors, such as nitric oxide[4], calcium-channel blockers[5], protein kinases[6] and bicarbonate radicals[7] were also studied. However, the exact mechanism underlying the tolerance and dependence is still unknown.
Neuropeptide Y (NPY) is a polypeptide of 36 amino acid residues widely distributed in the nervous system. This neuropeptide regulates various functions such as food uptake, blood pressure, circadian rhythm and anxiety. It induces the central and peripheral activities through at least six receptor subtypes called Y1−Y6 that belong to the G-protein coupled receptor superfamily[8].
After a period of BDZ treatment, the glutamatergic system was modified, which was thought to be one of the reasons underlying tolerance to BDZ. For example, an increase of 206% in in vitro glutamate release was found in the hippocampus of animals chronically treated with lorazepam[2]. Based on the facts that the NPYergic system inhibited the release of glutamate from presynaptic membranes[9] and that NPY was reduced in the CNS in alcohol dependence[10], the changes of NPYergic system in rat models with BDZ tolerance and dependence attracted our attention.
In this study, rat models of anticonvulsant tolerance and dependence to flurazepam (FZP, a BDZ) were established and the changes of NPY and its receptors (Y1, Y2, and Y5) in the hippocampus of these rat models were investigated.
Materials and methods
The establishment of rat models and the experiments on rats in this study were approved by the Ethics Committee of Animal Experiments of Peking University First Hospital.
Rat model of FZP tolerance The rat model of FZP tolerance used was produced by following the method developed by Rosenberg[11]. Male Sprague-Dawley rats (initial weight 180–200 g) were housed in a climate-controlled room with free access to standard rat food. FZP dissolved in a 0.02% saccharin solution was given as drinking water for 1 week (FZP-tolerant group, n=10). The concentration of FZP was adjusted to provide a daily dose of up to 100 mg/kg for the first 3 d and 150 mg/kg for the next 4 d. All rats consumed an average concentration of more than 100 mg/kg FZP daily. Control rats (n=10) were handled identically, but received 0.02% saccharin solution without the drug. After one week of treatment, rats were given saccharin solution as drinking water. Withdrawal signs such as overt ataxia, anxiety and hyperactivity were not found with this treatment[12].
Tolerance to FZP was evaluated 12 h after discontinuation of FZP intake. Because of the very rapid biotransformation of FZP and its metabolites in rats, and the corresponding plasma half-life (T1/2) of less than 2 h, the residual drug should not interfere with the anticonvulsant test at this time[13]. FZP dissolved in distilled water (100 mg/mL) was diluted to 20 mg/mL with normal saline for injection. This solution was injected intraperitoneally to deliver a dose of 20 mg/kg. After 30 min, 20 mg/mL pentylenetetrazol (PTZ, freshly prepared in normal saline) was infused at a constant rate of 0.5 mL/min into a tail vein, and clonus of the front legs was monitored to determine the threshold of PTZ-induced seizures. The onset time of clonus was recorded, and the PTZ threshold (mg/kg) was then derived. Rats in the control group were tested along with those in the FZP-tolerant group using the same procedure. FZP tolerance was determined by the decrease of PTZ threshold as compared with that of the control group. After the PTZ threshold test, the brain was removed and stored at -70 ºC immediately.
Rat model of FZP dependence We integrated the methods reported by Rosenberg[11] and Izzo et al[14] to establish a rat model of FZP dependence. The animals used, drug dosage, method of drug intake and PTZ threshold test were from the method reported by Rosenberg[11], but the days of drug administration and the dependence test were from the method reported by Izzo et al[14]. To obtain a more stable blood concentration, male Sprague-Dawley rats were given FZP dissolved in 0.02% saccharin solution as drinking water instead of an oral gavage of diazepam three times daily[14]. For rats in the FZP-dependent group (n=10), FZP was given at increasing doses for 14 d: d 1–3, 100 mg·kg-1·d-1; d 4–7, 150 mg·kg-1·d-1; d 8–10, 200 mg·kg-1·d-1; and d 11–14, 250 mg·kg-1·d-1. Control rats (n=10) were handled identically, but received 0.02% saccharin solution without the drug as drinking water. The emergence of dependence was determined by the susceptibility to PTZ-induced seizures 96 h after termination of the 14-day FZP administration. PTZ (20 mg/mL) was infused at a constant rate of 0.5 mL/min into a tail vein and the infusion was discontinued at the first sign of tonic-clonic seizures. The PTZ threshold (mg/kg) was derived from the amount of PTZ used to induce tonic-clonic seizures. Rats in the control group were treated identically. FZP dependence was determined by the decrease of the PTZ threshold as compared with that of the control group. After the PTZ threshold test, the brain was removed and stored at -70 ºC immediately.
PTZ-induced seizures themselves may also cause changes in the brain. To minimize these interferences, we killed rats immediately after the PTZ threshold test and compared the changes in the hippocampus with those from the respective control rats.
Reagents FZP was purchased from DaZhong Pharmaceutical (Shanghai, China).
Rats were from the Animal Department of Peking University Health Sciences Center. PTZ and anti-NPY antibody were from Sigma-Aldrich (Saint Louis, MO, USA). RNase-free DNase I was obtained from Promega (Madison, WI, USA), Trizol reagent and M-MuLV reverse transcriptase were from Gibco (Rockville, MD, USA). The sequences of oligonucleotide primers for the amplification of preproNPY (386 bp), Y1 (426 bp), Y2 (393 bp), and Y5 (391 bp) cDNA are: NPY-F, 5'- TATCCCTGCTCGTGTGTTTG-3'; NPY-R, 5'- AACGACAA-CAAGGGAAATGG-3'; Y1-F, 5'- ACTCTCACAGGCTGTC-TTAC-3'; Y1-R, 5'- ATAGTCTCGTAGTCGTCGTC-3'; Y2-F, 5'-AGCCTTTCCACCCTGCTAAT-3'; Y2-R, 5'-GTGAATGGCA-TCCAACCTCT-3'; Y5-F, 5'-CACCTAGCCGTTCCAGAA-AA-3'; Y5-R, 5'- GGGCTCTCAAGTCTGCTTTG-3'.
Measurement of preproNPY and NPY receptor cDNA in hippocampus by competitive RT-PCR Animals were decapitated immediately after the PTZ threshold test. The hippocampus was quickly removed and stored at -70 ºC until use. Brain tissue was homogenized in Trizol reagent in a pre-cooled mortar following manufacturer’s protocol[15]. Total RNA samples were treated with RNase-free DNase I to eliminate genomic DNA contamination before reverse transcrip-tion. Total RNA of 3 µg was reversely transcribed into cDNA using oligo d (T)15 as the primer and M-MuLV reverse transcriptase.
The internal competitive standards for the measurement of preproNPY, Y1, Y2, and Y5 cDNA were made using PCR. These DNA fragments had the same sequences as their respective PCR products, except that approximately 80 bp at the downstream site of the forward primers were deleted. Quantitative PCR was performed in a 0.2 mL tube containing brain cDNA 1 µL, internal competitive standard 1 µL, 5 µmol/L each primer mixture 1µL, 2.5 mmol/L each dNTP mixture 1 µL, 1.5 mmol/L MgCl2, 10×PCR buffer 2.5 µL, and 1.25 unit Taq DNA polymerase, in a total volume of 25 µL. PCR was run at 94 ºC 30 s, 62 ºC 30 s, and 72 ºC 40 s (93 ºC 1 min, 65 ºC 2 min and 72 ºC 2 min for the amplification of Y1 cDNA) for 35 cycles. PCR products were separated in a 1.5% agarose gel and stained using ethidium bromide. The ratio of the optical density of the two DNA bands under UV light was measured by a gel image analysis system. A standard curve of the ratio was drawn using the same conditions as described above, except that brain cDNA was changed to a serial dilution of the purified PCR product. The relative amount of the cDNA in a sample was then obtained from the standard curve.
Immunohistochemistry of NPY in hippocampus After anesthesia, rats were perfused through the left ventricle with 0.9% sodium chloride followed by 4% paraformaldehyde in phosphate buffer solution for approximately 30 min. The removed brain was sequentially soaked in 4% paraformaldehyde in phosphate buffer solution for 24 h and 30% sucrose at 4 ºC for 72 h. Frozen sections of 8-mm thickness in a coronal plane were used for immunohistochemistry. One brain section from the treated rat and one from the respective control rat were placed on one slide and processed together. After treatment with 0.3% H2O2 to block the endogenous peroxidase, sections were incubated with 1:50 polyclonal anti-NPY antibody overnight at 4 ºC. The secondary antibody was biotinylated anti-rabbit IgG antibody (Vector, Burlingame, CA, USA). Signals were visualized by horseradish peroxidase conjugated avidin and diamino-benzidine. The relative density of NPY-immunoreactive material in neurons in the pyramidal cell layer of CA1, CA3 region and granular cell layer of the dentate gyrus region was measured in a defined area by a densitometer.
Statistical analysis Data were expressed as mean±SD. Paired t-tests were used to evaluate the significance of intergroup differences. P<0.05 was considered as significant.
Results
Rat models of FZP tolerance and dependence
FZP-tolerant model The rat model of FZP tolerance was evaluated by studying the FZP anticonvulsant efficacy after discontinuing the 7-d FZP treatment for 12 h when the brain FZP should have been metabolized[13]. Anticonvulsive efficacy of a single dose of 20 mg/kg FZP was measured by studying the threshold of PTZ-induced seizures after 30 min. In comparison with the control group, the average PTZ threshold decreased by more than 3 times in the FZP-tolerant group, indicating the successful establishment of an FZP-tolerant model in rats (Table 1).

Full table
FZP-dependent model In contrast to the evaluation of FZP tolerance, FZP dependence was estimated by studying the PTZ threshold after discontinuing the 14-d FZP treatment for 96 h when maximum withdrawal signs were expected to occur[14]. As shown in Table 1, the average PTZ threshold in the FZP-dependent model was lower than that in its control group by more than 2 times, indicating that the FZP-dependent model is acceptable for further study (Table 1).
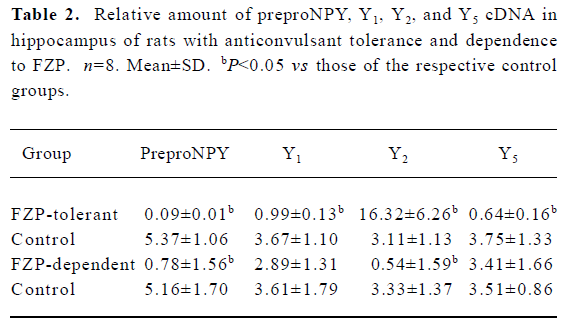
Changes of preproNPY cDNA in hippocampus of FZP-tolerant and -dependent rats In the hippocampus, preproNPY cDNA was dramatically reduced both in FZP-tolerant and -dependent groups, as compared with that of the respective control groups (Table 2, Figure 1).
Full table

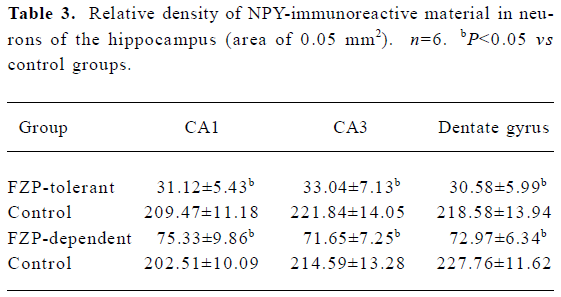
NPY-immunoreactive cells were found in many brain regions, with the hippocampus showing the most remarkable changes in FZP tolerant and dependent rats. In the hippocampus of control animals, NPY-immunoreactive cells were mostly located in CA1, CA3, and dentate gyrus regions. NPY-immunoreactive cells were large multipolar or fusiform neurons, and NPY positive material was found in their cytoplasm and processes (Figure 2A, 2B). In the hippocampus from FZP-tolerant and FZP-dependent rats, NPY-immunoreactive material in the cytoplasm and processes was significantly reduced as compared with that in the same regions of the respective control rats (Figure 2C, 2D, Table 3).

Full table
Changes of NPY receptor cDNA in hippocampus of FZP-tolerant and -dependent rats Y1 and Y5 cDNA were decreased in the tolerant group as compared with those of the respective control group. However, no quantitative changes of these two cDNA were found in the FZP-dependent group. In contrast, Y2 cDNA was increased significantly in the FZP-tolerant group, but was decreased in the FZP-dependent group, as compared with that of the respective controls (Table 2, Figure 1).
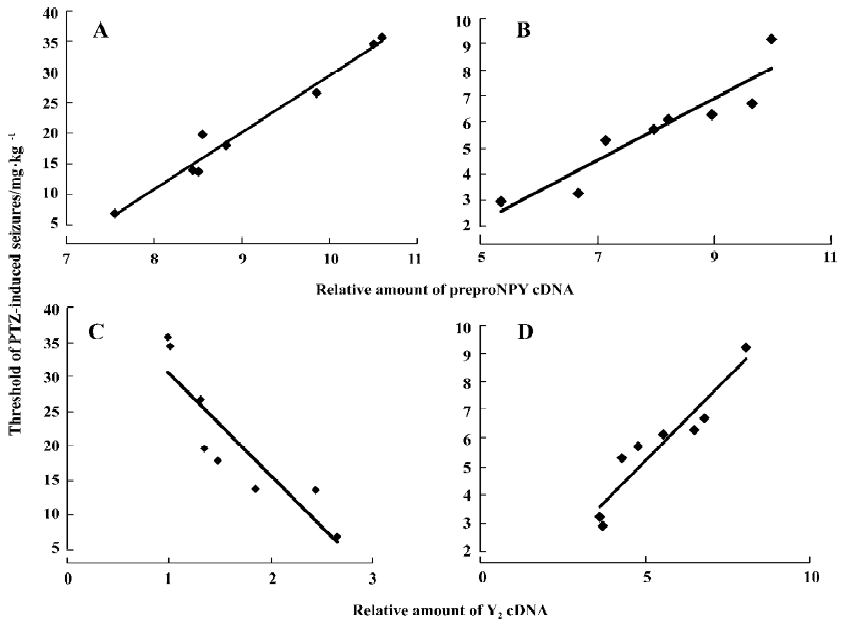
Correlations between the threshold of PTZ-induced seizures and the levels of preproNPY, Y1, Y2, and Y5 cDNA in hippocampus of FZP-tolerant and -dependent rats The correlations were examined to show whether changes of NPY and its receptors in the hippocampus were related to the pathogenesis of FZP tolerance and dependence. The PTZ threshold was positively correlated with the level of preproNPY cDNA in the hippocampus both in FZP-tolerant and -dependent groups (Figure 3A, 3B). In other words, the lower the expression of NPY in the hippocampus, the higher degree of tolerance and dependence the rat showed. The PTZ threshold was negatively correlated with the level of hippocampal Y2 cDNA in the FZP-tolerant group and was positively correlated with the level of hippocampal Y2 cDNA in the FZP-dependent group (Figure 3C, 3D). No correlation between the PTZ threshold and the changes of Y1 and Y5 cDNA were found (data not shown).
Discussion
Pharmacologically, BDZ produce their sedative, anxiolytic and antiepileptic activities through a modulation action on GABAA receptors. However, tolerance to BDZ and dependence on BDZ are the two major adverse reactions in clinical practice. Neuronal hyperexcitability may be the common basis for the tolerance and dependence, although they may have different manifestations.
Hippocampus is implicated in the generation and modulation of seizure activities, and plays a central role in controlling excitability of the CNS[16]. In the present study, the hippocampus was separated to investigate the changes of NPY and its receptors (Y1, Y2, and Y5) in rat models with anticonvulsant tolerance and dependence to FZP.
We found that preproNPY cDNA in the hippocampus was significantly decreased in both the FZP-tolerant and FZP-dependent groups. Immunohistochemistry also showed the reduction of NPY-immunoreactive material in the neuronal cytoplasm and neurofibers of CA1, CA3, and dentate gyrus regions. Y1 and Y5 receptor cDNA were decreased in the tolerant group but were not changed in the dependent group. Y2 receptor cDNA was increased in the tolerant group but was decreased in the dependent group.
To date, only one paper has been published on the relationship between the NPYergic system and drug tolerance and/or dependence[17]: contingent tolerance to carbamazepine after repeatedly giving the drug may be associated with the down-regulation of NPY. However, no data about the changes of the NPYergic system following long-term treatment of BDZ have been found in the published literature.
The level of preproNPY cDNA in the hippocampus reversely relating to the degree of tolerance and dependence may suggest that the changes of preproNPY cDNA are involved in the pathogenesis of FZP tolerance and dependence. NPY, one of the most abundant and widely distributed neuropeptides in the central and peripheral nervous systems, acts as an endogenous modulator to reduce seizure activities. In the hippocampus, the inhibition of epileptiform discharges after NPY treatment was attributed to the decrease of glutamate released from the presynaptic nerve terminals through blocking of the G-protein dependent calcium channel[18]. Therefore, the decrease of cellular NPY we found may result in neuronal hyperexcitability that is the fundamental pathological state of BDZ tolerance and dependence.
Six subtypes of NPY receptors, Y1−Y6, have been identified. Their pharmacological characteristics are very similar, although the identity of amino acid sequences is as low as 30% among these receptor subtypes[19]. Of the six receptor subtypes, Y1, Y2, and Y5 are the major functional receptors and are expressed abundantly. In rodents, changes in the Y1 receptor and its mRNA in the CNS can be induced by various stimuli, suggesting the multiple functions of the Y1 receptor[20]. The Y2 receptor demonstrates a predominant role in modulation of seizure activity[21]. The Y5 receptor may also be involved in the modulation of anticonvulsant activity in rodents[22,23]. Theoretically, drug tolerance and dependence are caused by the imbalance of excitation over inhibition in the brain, and NPY receptors would decrease in association with the decrease of NPY. The different changes to NPY receptors between the FZP-tolerant and FZP-dependent group and the increase of Y2 in FZP-tolerant rats in this study can not be satisfactorily explained because of the insufficient and conflicting data regarding Y1, Y2, and Y5 receptors in the regulation of neuronal excitability at the present time. More experiments should be performed on the distribution and ligand binding capacity of the three subtype receptors in FZP tolerance and dependence.
Our preliminary results suggest that the decrease of NPY in the hippocampus plays an important role in the generation of anticonvulsant tolerance and dependence following long-term FZP treatment. The NPYergic system may be a new target for the comprehension of these adverse effects of BDZ.
References
- Brown MJ, Wood MD, Coldwell MC, Bristow DR. Gamma-aminobutyric acid A receptor function is desensitised in rat cultured cerebellar granule cells following chronic flunitrazepam treatment. J Neurochem 1998;71:1232-40.
- Bonavita C, Ferrero A, Cereseto M, Velardez M, Rubio M, Wikinski S. Adaptive changes in the rat hippocampal gluta-matergic neurotransmission are observed during long-term treatment with lorazepam. Psychopharmacology (Berl) 2003;166:163-7.
- Byrnes JJ, Miller LG, Perkins K, Greenblatt DJ, Shader RI. Chronic benzodiazepine administration. XI. Concurrent administration of PK11195 attenuates lorazepam discontinuation effects. Neuropsychopharmacology 1993;8:267-73.
- Gupta N, Bhargava VK, Pandhi P. Tolerance and withdrawal to anticonvulsant action of clonazepam: role of nitric oxide. Methods Find Exp Clin Pharmacol 2000;22:229-35.
- Mizoguchi H, Yoshiike M, Suzuki T, Misawa M. Effects of Ca2+ channel blockers on physical dependence on diazepam in mice. Life Sci 1993;53:PL365-70.
- Lilly SM, Zeng XJ, Tietz EI. Role of protein kinase A in GABAA receptor dysfunction in CA1 pyramidal cells following chronic benzodiazepine treatment. J Neurochem 2003;85:988-98.
- Zeng XJ, Tietz EI. Role of bicarbonate ion in mediating decreased synaptic conductance in benzodiazepine tolerant hippocampal CA1 pyramidal neurons. Brain Res 2000;868:202-14.
- Blomqvist AG, Herzog H. Y-receptor subtypes — how many more? Trends Neurosci 1997;20:294-8.
- Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y action in the rat hippocampal slice: site and mechanism of presynaptic inhibition. J Neurosci 1988;8:3827-37.
- Bison S, Crews F. Alcohol withdrawal increases neuropeptide Y immunoreactivity in rat brain. Alcohol Clin Exp Res 2003;27:1173-83.
- Rosenberg HC. Differential expression of benzodiazepine anticonvulsant cross-tolerance according to time following flurazepam or diazepam treatment. Pharmacol Biochem Behav 1995;51:363-8.
- Rosenberg HC, Chiu TH. Tolerance during chronic benzodiazepine treatment associated with decreased receptor binding. Eur J Pharmacol 1981;70:453-60.
- Lau CE, Falk JL, Dolan S, Tang M. Simultaneous determination of flurazepam and five metabolites in serum by high-performance liquid chromatography and its application to pharmacokinetic studies in rats. J Chromatogr 1987;423:251-9.
- Izzo E, Auta J, Impagnatiello F, Pesold C, Guidotti A, Costa E. Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. Proc Natl Acad Sci USA 2001;98:3483-8.
- Sammbrook J, Fritsch EF, Maniatis T. Molecular cloning, a laboratory manual, 3rd Ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2001. p 8.86–9.
- Loscher W, Ebert U. The role of the piriform cortex in kindling. Prog Neurobiol 1996;50:427-81.
- Weiss SR, Clark M, Rosen JB, Smith MA, Post RM. Contingent tolerance to the anticonvulsant effects of carbamazepine: relationship to loss of endogenous adaptive mechanisms. Brain Res Brain Res Rev 1995;20:305-25.
- Qian J, Colmers WF, Saggau P. Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry. J Neurosci 1997;17:8169-77.
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci 1999;11:1431-48.
- Gariboldi M, Conti M, Cavaleri D, Samanin R, Vezzani A. Anticonvulsant properties of BIBP3226, a non-peptide selective antagonist at neuropeptide Y Y1 receptors. Eur J Neurosci 1998;10:757-9.
- Kaga T, Fujimiya M, Inui A. Emerging functions of neuropeptide Y Y(2) receptors in the brain. Peptides 2001;22:501-6.
- Woldbye DP, Larsen PJ, Mikkelsen JD, Klemp K, Madsen TM, Bolwig TG. Powerful inhibition of kainic acid seizures by neuropeptide Y via Y5-like receptors. Nat Med 1997;3:761-4.
- Wu YF, Li SB. Neuropeptide Y expression in mouse hippocampus and its role in neuronal excitotoxicity. Acta Pharmacol Sin 2005;26:63-8.
