Recent progress in α1-adrenergic receptor research
Introduction
α1-Adrenergic receptors (AR) are members of the G protein-coupled receptor (GPCR) superfamily that mediate physiological responses to norepinephrine (NE) and epinephrine (EPI). Pharmacological analysis and molecular cloning have shown that this receptor family has three subtypes (α1A, α1B, α1D), which have different pharmacological properties and amino acid sequences[1]. Four C-terminal splice variants of the α1A-AR have been found[2,3]. Stimulation of all three subtypes results in activation of the Gq/11 signaling pathway, involving activation of phospholipase C, generation of the second messengers inositol (1,4,5) triphosphate and diacylglycerol, and mobilization of intracellular calcium. Because of their clinical importance, research on α1-AR has been very active for many years. Several recent reviews have summarized this research from different perspectives[4–9]. In this review, we mainly focus on recent developments in subtype-selective drugs, genetically engineered mouse models, interacting proteins, receptor dimerization, and factors controlling receptor cell surface expression.
α1-AR subtype selective drugs
Although all three α1-AR subtypes activate the same Gq/11 protein signaling pathway, their different tissue distributions suggest that they play distinct functional roles. For example, the α1A-AR subtype has been considered to be the dominant receptor subtype controlling benign prostatic hypertrophy, and is an important therapeutic target for treatment of this disease[10]. Therefore, identifying the specific functions of individual α1-AR subtypes is of considerable therapeutic interest. Unfortunately, this has proved difficult because of difficulties in identifying highly subtype-selective drugs. Despite extensive efforts to address this difficulty, only a few subtype-selective compounds have been characterized[11,12]. WB 4101[13], (+)-niguldipine[14], and 5-methylurapidil[15] have been used extensively as α1A-AR selective antagonists, and BMY7378[16] is widely used to characterize α1D-AR. However, there has been little progress in identifying α1B-AR selective antagonists. Chlorethyclonidine was originally characterized as an α1B-AR selective site-directed alkylating agent[17]; however, it has limited selectivity in alkylating the cloned subtypes, and is no longer widely used. Recently, a conopeptide isolated from a sea snail, known as ρ-TIA, was identified as a non-competitive α1B-AR antagonist[18,19] that may serve as an allosteric modulator, but acts as a competitive inhibitor at the other two subtypes[20,21]. This suggests that ρ-TIA may interact with a novel antagonist binding site specific to the α1B-AR and serve as a template for development of highly selective α1B-AR drugs.
Genetically engineered mouse models
Since attempts to elucidate the function of individual receptor subtypes has been limited by a lack of agonists, antagonists, and antibodies with adequate subtype selectivity, recent studies using genetically engineered mice have shed some light on the contributions of each subtype to physiological responses to NE and EPI. In general, α1-AR knockout mice do not show overt physical abnormalities (Table 1). Studies in knockout mice lacking a single α1-AR subtype have shown that all three subtypes seem to be involved in the regulation of blood pressure. Consistent with previous pharmacological characterization in vitro, studies in α1D-AR knockout mice have shown that the α1D-AR subtype plays a dominant role in aortic contraction[22]. Not only do genetically engineered mouse models help clear up some of the confusing cardiovascular effects mediated by individual subtypes, they also provide clues as to the functional roles of each subtype in the central nervous system, where these receptors are expressed in high concentrations, but their functions have been difficult to determine. The involvement of α1B-AR in the regulation of locomotion has been suggested by pharmacological manipulation[23]. Interestingly, knockout mice lacking α1B-AR also showed decreased locomotor hyperactivity in response to psychostimulants and opiates, suggesting that targeting the α1B-AR might be a useful therapeutic strategy in the treatment of drug abuse[24]. In addition to the altered phenotypes being examined in the knockout or transgenic models, the underlying molecular mechanisms have been investigated using olignucleotide microarrays. Alteration in the expression of NMDA receptors, GABAA receptors, and apoptotic and calcium regulatory genes shown in transgenic murine brains may provide a potential molecular basis for neurodegeneration induced by overexpressed constitutively active α1B-AR[25]. Despite the new insights provided by the genetically engineered studies, some discrepancies have been noticed from different studies, such as the contribution of α1B-AR in the aortic contractile response[26,27]. In addition, some of the altered phenotypes observed in the α1B-AR knockout model may result from compensatory effects of other receptor subtypes[28]. Further investigation using classic pharmacological approaches with highly subtype-selective drugs would be useful to facilitate future interpretations of those data.
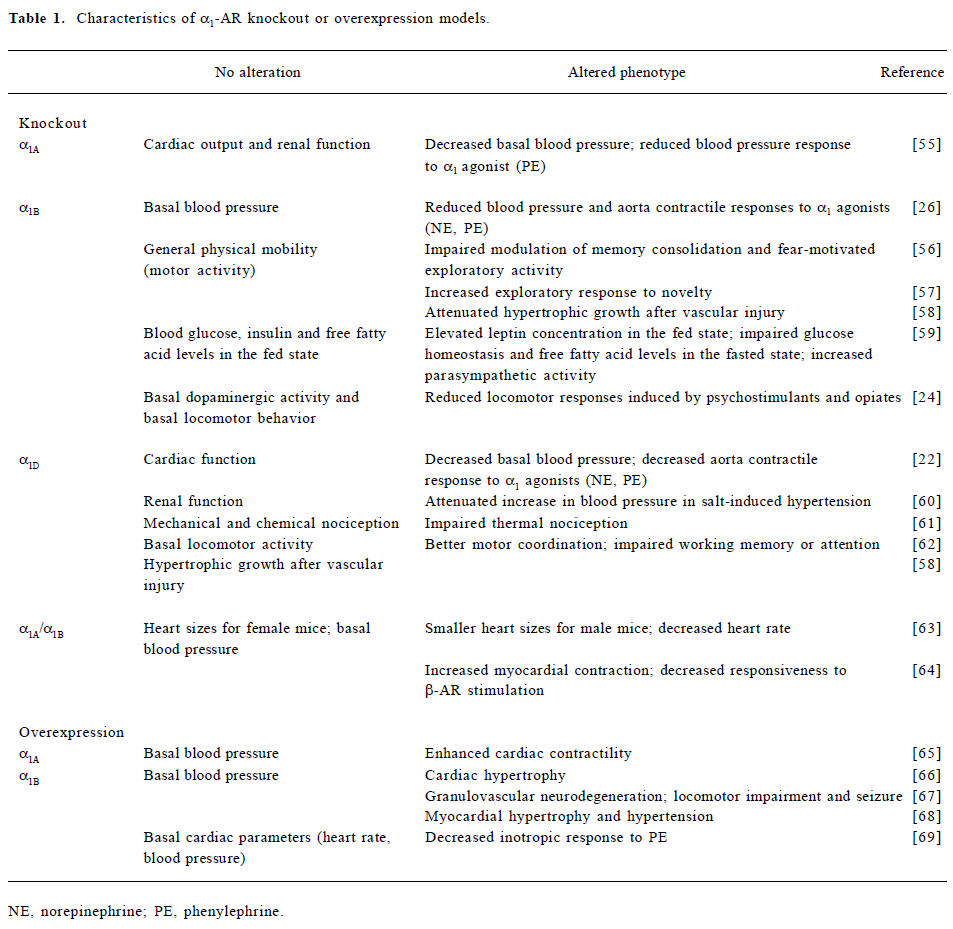
Full table
Receptor interacting proteins
Recent studies have revealed that GPCR can interact with various cellular proteins in addition to the cognate G proteins, thereby expanding the receptor signaling network and establishing the distinct functional roles of closely-related receptor subtypes within the same family[29,30]. Those interacting proteins include cytoplasmic and membrane proteins that may play regulatory roles in receptor pharmacology, trafficking and signaling[31]. Although all three α1-AR subtypes couple to Gq/11 signaling pathways, previous studies have shown that the three subtypes can activate distinct downstream signaling components in the Gq/11 signaling pathway or couple to different signaling pathways[1]. Because of their relatively long C-termini, which have the least sequence homology among the three subtypes, most attention has been focused on finding binding partners interacting with this region. In addition, the sequence diversity in the third intracellular loop (I3 loop) is also attractive due to its importance in coupling to Gq/11. In a similar manner, it has been shown that the three β-AR subtypes differentially associate with a variety of proteins other than G proteins[32]. However, only a handful of interacting proteins have been identified for α1-AR subtypes (Table 2). A few of these interactions have been shown to have functional consequences, but most of them require further evaluation. For example, gC1qR and the mu2 subunit of the AP2 clathrin adaptor complex are involved in α1B-AR trafficking or internalization. Although transglutaminase II (Gh) is the first non-Gq/11 binding partner found to specifically associate with the α1B- and α1D-AR[33,34], the α1-AR signaling pathways seem to remain intact in Gh knockout mice[35]. Nevertheless, the search for novel binding partners should be considered as an alternative approach to study the molecular differences among the three α1-AR subtypes.
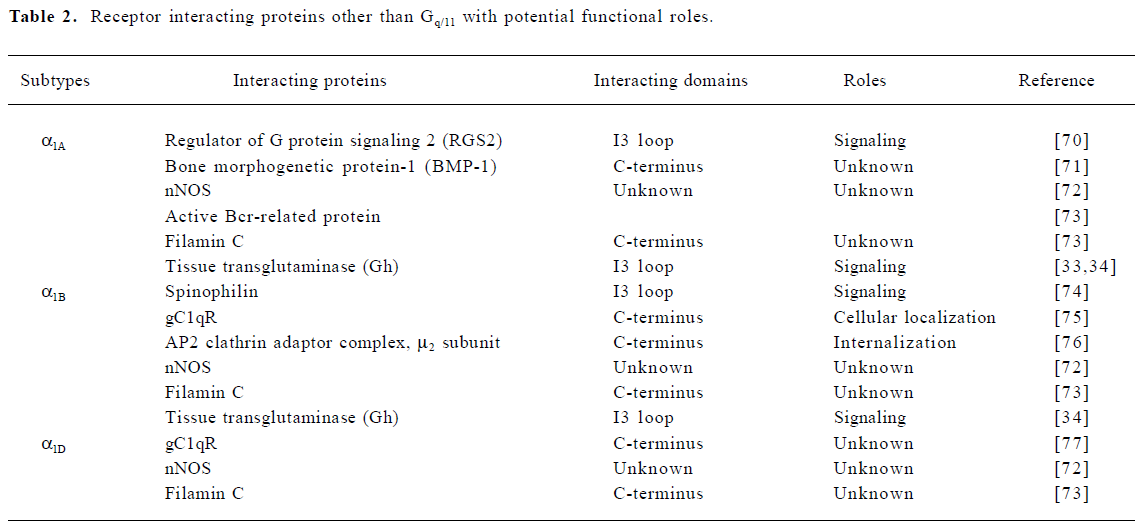
Full table
Receptor dimerization
Unlike the conventional view that GPCR are monomers, a growing body of evidence indicates that GPCR are able to form dimers or oligomers that are required for their pharmacology, function and/or cell surface expression. This concept has gained great appreciation for the class III GPCR subfamily, including the GABAB receptors[36,37] and taste receptors[38]. However, the significance of class I GPCR dimerization has been under debate, due to difficulties in identifying unique functional responses or pharmacological properties. Our group has shown that the three α1-AR subtypes can form homodimers and subtype-specific heterodimers with other AR[39,40], which has been confirmed by data from other groups[41,42]. Because truncation of either the amino or the carboxyl terminus of the receptor does not affect receptor dimerization[40], the transmembrane domains or associated loops have been proposed to be involved in this interaction. Because the pharmacological analyses of the three cloned α1-AR subtypes in heterologous expression systems have not yet recapitulated all the receptor subtypes previously defined by pharmacological criteria in tissues, such as the α1L-AR[43], the newly found receptor dimers have been hypothesized to perform atypical α1-AR pharmacology, which has been seen in the opioid receptor family[44]. Although the homo- or heterodimers formed by α1A-AR C-terminal splice variants have failed to show any novel pharmacology when studied with existing selective drugs[45], it is still hard to conclude that the dimerization does not have effects on receptor pharmacology, because the available drugs are limited and the particular receptor dimers may yet be identified. On the other hand, the α1-AR subtype-specific dimerization has been found to be important for receptor trafficking and signaling (summarized in the next section).
α1D-AR cell surface expression
Theoretically, all three α1-AR subtypes should be present at the cell surface to be recognized by their highly hydrophilic natural ligands that are unlikely to cross cell membranes. However, when expressed in recombinant systems, the α1D-AR subtype has been noted to show almost exclusively intracellular expression[46,47], which makes them difficult to characterize. We recently reported that cell surface expression of the α1D-AR could be specifically rescued by coexpression with the α1B-AR but not the α1A-AR[48]. The coexpressed receptors seem to form a new receptor entity, and then modulate signaling and internalization of each receptor subtype in the complex. Recently, the β2-AR was also reported to be able to translocate α1D-AR[49]. Besides receptor dimerization, removal of the long amino-terminus of the α1D-AR has also been shown to facilitate translocation of intracellular receptors to the cell surface[50]. Subsequent studies using receptor N-terminal chimeras showed that this N-terminal domain might convey a retention signal to prevent receptor cell surface expression[51]. Moreover, the density of α1D-AR cell surface expression was shown to increase upon sequential truncation[52].
Future directions
In the past few years, our knowledge of the functional roles of the α1-AR family has been dramatically expanded using many different approaches. These findings generate several interesting directions that may be worth pursuing.
1 Further development of highly subtype-selective drugs, especially non-competitive antagonists: most of the specific α1-AR drugs available are competitive antagonists with moderate subtype-selectivity, which also target other cell surface proteins. Since the three α1-AR subtypes have relatively high homologies among the transmembrane domains that are believed to form the ligand binding pocket, designing new subtype-selective drugs that compete for this site has not been easy. However, development of noncompetitive drugs may be a good strategy because those drugs normally recognize a different site with less homology. This strategy has been successfully applied in designing highly subtype-selective drugs for other GPCR[53].
2 Investigate the physiological relevance of receptor oligomers: the success of this direction requires the advance of two other research fields, characterization of subtype-specific antibodies and development of new α1-AR drugs. Although receptor dimerization and protein–protein interactions have been identified in recombinant systems, and their importance in advancing our knowledge of the α1-AR family has been recognized, their physiological relevance in vivo cannot be confirmed and exploited without subtype-specific antibodies. On the other hand, new subtype-selective drugs may recognize the discrete pharmacology of receptor dimers, therefore expanding the existing cloned α1-AR family and providing new therapeutic targets.
3 Characterize subtype-specific interacting proteins: the growing list of GPCR interacting proteins has elucidated the molecular mechanisms of the differences among subtypes, which could lead to the development of drugs specifically targeting to such interactions. Because previous evidence suggests that the three α1-AR subtypes might couple to different signaling pathways, it is likely that more interacting proteins would be identified through the approaches that have been successfully used, such as yeast two-hybrid screenings and pull-down assays with fusion proteins.
4 Study the functional role of α1-AR in the central nervous system[5]. In fact, almost all typical and atypical antipsychotics are α1-AR antagonists, although they show little, if any, subtype selectivity[54]. In addition, tricyclic antidepressants are also α1-AR antagonists, and it is possible that this property may contribute to their therapeutic efficacy. We believe that studies focusing on those directions would further improve our understanding of the functional roles of each α1-AR subtype.
References
- Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol 1999;375:261-76.
- Hirasawa A, Shibata K, Horie K, Takei Y, Obika K, Tanaka T, et al. Cloning, functional expression and tissue distribution of human alpha 1c-adrenoceptor splice variants. FEBS Lett 1995;363:256-60.
- Chang DJ, Chang TK, Yamanishi SS, Salazar FH, Kosaka AH, Khare R, et al. Molecular cloning, genomic characterization and expression of novel human alpha1A-adrenoceptor isoforms. FEBS Lett 1998;422:279-83.
- Piascik MT, Perez DM. Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther 2001;298:403-10.
- Pupo AS, Minneman KP. Adrenergic pharmacology: focus on the central nervous system. CNS Spectrums 2001;6:656-62.
- Tanoue A, Koshimizu TA, Tsujimoto G. Transgenic studies of alpha(1)-adrenergic receptor subtype function. Life Sci 2002;71:2207-15.
- Hague C, Chen Z, Uberti M, Minneman KP. alpha(1)-Adrenergic receptor subtypes: non-identical triplets with different dancing partners? Life Sci 2003;74:411-8.
- Koshimizu TA, Tanoue A, Hirasawa A, Yamauchi J, Tsujimoto G. Recent advances in alpha1-adrenoceptor pharmacology. Pharmacol Ther 2003;98:235-44.
- Hawrylyshyn KA, Michelotti GA, Coge F, Guenin SP, Schwinn DA. Update on human alpha1-adrenoceptor subtype signaling and genomic organization. Trends Pharmacol Sci 2004;25:449-55.
- Nagarathnam D, Wetzel JM, Miao SW, Marzabadi MR, Chiu G, Wong WC, et al. Design and synthesis of novel alpha1a adrenoceptor-selective dihydropyridine antagonists for the treatment of benign prostatic hyperplasia. J Med Chem 1998;41:5320-33.
- Hancock AA. alpha1-adrenoceptor subtypes: a synopsis of their pharmacology and molecular biology. Drug Dev Res 1996;39:54-107.
- Romeo G, Materia L, Salerno L, Russo F, Minneman KP. Novel antagonists for alpha1-adrenoceptor subtypes. Expert Opin Ther Patents 2004; 14: 619–37.
- Morrow AL, Creese I. Characterization of alpha 1-adrenergic receptor subtypes in rat brain: a reevaluation of [3H]WB4104 and [3H]prazosin binding. Mol Pharmacol 1986;29:321-30.
- Boer R, Grassegger A, Schudt C, Glossmann H. (+)-Niguldipine binds with very high affinity to Ca2+ channels and to a subtype of alpha 1-adrenoceptors. Eur J Pharmacol 1989;172:131-45.
- Gross G, Hanft G, Rugevics C. 5-Methyl-urapidil discriminates between subtypes of the alpha 1-adrenoceptor. Eur J Pharmacol 1988;151:333-5.
- Goetz AS, King HK, Ward SD, True TA, Rimele TJ, Saussy DL. BMY 7378 is a selective antagonist of the D subtype of alpha 1-adrenoceptors. Eur J Pharmacol 1995;272:R5-6.
- Han C, Abel PW, Minneman KP. Heterogeneity of alpha 1-adrenergic receptors revealed by chlorethylclonidine. Mol Pharmacol 1987;32:505-10.
- Sharpe IA, Gehrmann J, Loughnan ML, Thomas L, Adams DA, Atkins A, et al. Two new classes of conopeptides inhibit the alpha1-adrenoceptor and noradrenaline transporter. Nat Neurosci 2001;4:902-7.
- Sharpe IA, Thomas L, Loughnan M, Motin L, Palant E, Croker DE, et al. Allosteric alpha 1-adrenoreceptor antagonism by the conopeptide rho-TIA. J Biol Chem 2003;278:34451-7.
- Chen Z, Rogge G, Hague C, Alewood D, Colless B, Lewis RJ, et al. Subtype-selective noncompetitive or competitive inhibition of human alpha1-adrenergic receptors by rho-TIA. J Biol Chem 2004;279:35326-33.
- Lima V, Mueller A, Kamikihara SY, Raymundi V, Alewood D, Lewis RJ, et al. Differential antagonism by conotoxin rho-TIA of contractions mediated by distinct alpha1-adrenoceptor subtypes in rat vas deferens, spleen and aorta. Eur J Pharmacol 2005;508:183-92.
- Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, et al. The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest 2002;109:765-75.
- Stone EA, Lin Y, Itteera A, Quartermain D. Pharmacological evidence for the role of central alpha 1B-adrenoceptors in the motor activity and spontaneous movement of mice. Neuropharmacology 2001;40:254-61.
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, et al. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci 2002;22:2873-84.
- Yun J, Gaivin RJ, McCune DF, Boongird A, Papay RS, Ying Z, et al. Gene expression profile of neurodegeneration induced by alpha1B-adrenergic receptor overactivity: NMDA/GABAA dysregulation and apoptosis. Brain 2003;126:2667-81.
- Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, et al. Decreased blood pressure response in mice deficient of the alpha1b-adrenergic receptor. Proc Natl Acad Sci USA 1997;94:11589-94.
- Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, et al. A knockout approach indicates a minor vasoconstrictor role for vascular alpha1B-adrenoceptors in mouse. Physiol Genomics 2002;9:85-91.
- Deighan C, Woollhead AM, Colston JF, McGrath JC. Hepatocytes from alpha1B-adrenoceptor knockout mice reveal compensatory adrenoceptor subtype substitution. Br J Pharmacol 2004;142:1031-7.
- Hall RA, Premont RT, Lefkowitz RJ. Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol 1999;145:927-32.
- Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res 2002;91:672-80.
- Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP). Pharmacol Ther 2004;103:203-21.
- Hall RA. Beta-adrenergic receptors and their interacting proteins. Semin Cell Dev Biol 2004;15:281-8.
- Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, et al. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science 1994;264:1593-6.
- Chen S, Lin F, Iismaa S, Lee KN, Birckbichler PJ, Graham RM. Alpha1-adrenergic receptor signaling via Gh is subtype specific and independent of its transglutaminase activity. J Biol Chem 1996;271:32385-91.
- Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem 2001;276:20673-8.
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 1998;396:679-82.
- Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC. Role of heteromer formation in GABAB receptor function. Science 1999;283:74-7.
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 2001;106:381-90.
- Vicentic A, Robeva A, Rogge G, Uberti M, Minneman KP. Biochemistry and pharmacology of epitope-tagged alpha(1)-adrenergic receptor subtypes. J Pharmacol Exp Ther 2002;302:58-65.
- Uberti MA, Hall RA, Minneman KP. Subtype-specific dimerization of alpha 1-adrenoceptors: effects on receptor expression and pharmacological properties. Mol Pharmacol 2003;64:1379-90.
- Stanasila L, Perez JB, Vogel H, Cotecchia S. Oligomerization of the alpha 1a- and alpha 1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J Biol Chem 2003;278:40239-51.
- Carrillo JJ, Pediani J, Milligan G. Dimers of class A G protein-coupled receptors function via agonist-mediated trans-activation of associated G proteins. J Biol Chem 2003;278:42578-87.
- Ohmura T, Sakamoto S, Hayashi H, Kigoshi S, Muramatsu I. Identification of alpha 1-adrenoceptor subtypes in the dog prostate. Urol Res 1993;21:211-5.
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimer-ization modulates receptor function. Nature 1999;399:697-700.
- Ramsay D, Carr IC, Pediani J, Lopez-Gimenez JF, Thurlow R, Fidock M, et al. High-affinity interactions between human alpha1A-adrenoceptor C-terminal splice variants produce homo- and heterodimers but do not generate the alpha1L-adrenoceptor. Mol Pharmacol 2004;66:228-39.
- McCune DF, Edelmann SE, Olges JR, Post GR, Waldrop BA, Waugh DJ, et al. Regulation of the cellular localization and signaling properties of the alpha(1B)- and alpha(1D)-adrenoceptors by agonists and inverse agonists. Mol Pharmacol 2000;57:659-66.
- Chalothorn D, McCune DF, Edelmann SE, Garcia-Cazarin ML, Tsujimoto G, Piascik MT. Differences in the cellular localization and agonist-mediated internalization properties of the alpha(1)-adrenoceptor subtypes. Mol Pharmacol 2002;61:1008-16.
- Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. Cell surface expression of alpha 1D-adrenergic receptors is controlled by heterodimerization with alpha 1B-adrenergic receptors. J Biol Chem 2004;279:15541-9.
- Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. Heterodimerization with {beta}2-adrenergic receptors promotes surface expression and functional activity of {alpha}1D-adrenergic receptors. J Pharmacol Exp Ther 2005;313:16-23.
- Pupo AS, Uberti MA, Minneman KP. N-terminal truncation of human alpha(1D)-adrenoceptors increases expression of binding sites but not protein. Eur J Pharmacol 2003;462:1-8.
- Hague C, Chen Z, Pupo AS, Schulte N, Toews ML, Minneman KP. The N-terminus of the human alpha-1D-adrenergic receptor prevents cell surface expression. J Pharmacol Exp Ther 2004;309:388-97.
- Petrovska R, Kapa I, Klovins J, Schioth HB, Uhlen S. Addition of a signal peptide sequence to the alpha(1D)-adrenoceptor gene increases the density of receptors, as determined by [(3)H]-prazosin binding in the membranes. Br J Pharmacol 2005;144:651-9.
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev 2002;54:323-74.
- Stone EA, Lin Y, Rosengarten H, Kramer HK, Quartermain D. Emerging evidence for a central epinephrine-innervated alpha 1-adrenergic system that regulates behavioral activation and is impaired in depression. Neuropsychopharmacology 2003;28:1387-99.
- Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA 2002;99:9474-9.
- Knauber J, Muller WE. Decreased exploratory activity and impaired passive avoidance behaviour in mice deficient for the alpha(1b)-adrenoceptor. Eur Neuropsychopharmacol 2000;10:423-7.
- Spreng M, Cotecchia S, Schenk F. A behavioral study of alpha-1b adrenergic receptor knockout mice: increased reaction to novelty and selectively reduced learning capacities. Neurobiol Learn Mem 2001;75:214-29.
- Zhang H, Cotecchia S, Thomas SA, Tanoue A, Tsujimoto G, Faber JE. Gene deletion of dopamine beta-hydroxylase and alpha1-adrenoceptors demonstrates involvement of catecholamines in vascular remodeling. Am J Physiol Heart Circ Physiol 2004;287:H2106-14.
- Burcelin R, Uldry M, Foretz M, Perrin C, Dacosta A, Nenniger-Tosato M, et al. Impaired glucose homeostasis in mice lacking the alpha1b-adrenergic receptor subtype. J Biol Chem 2004;279:1108-15.
- Tanoue A, Koba M, Miyawaki S, Koshimizu TA, Hosoda C, Oshikawa S, et al. Role of the alpha1D-adrenergic receptor in the development of salt-induced hypertension. Hypertension 2002;40:101-6.
- Harasawa I, Honda K, Tanoue A, Shinoura H, Ishida Y, Okamura H, et al. Responses to noxious stimuli in mice lacking alpha(1d)-adrenergic receptors. Neuroreport 2003;14:1857-60.
- Mishima K, Tanoue A, Tsuda M, Hasebe N, Fukue Y, Egashira N, et al. Characteristics of behavioral abnormalities in alpha1d-adrenoceptors deficient mice. Behav Brain Res 2004;152:365-73.
- O’Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, et al. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest 2003;111:1783-91.
- McCloskey DT, Turnbull L, Swigart P, O’Connell TD, Simpson PC, Baker AJ. Abnormal myocardial contraction in alpha(1A)- and alpha(1B)-adrenoceptor double-knockout mice. J Mol Cell Cardiol 2003;35:1207-16.
- Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, Arthur JF, et al. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res 2001;89:343-50.
- Milano CA, Dolber PC, Rockman HA, Bond RA, Venable ME, Allen LF, et al. Myocardial expression of a constitutively active alpha 1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc Natl Acad Sci USA 1994;91:10109-13.
- Zuscik MJ, Sands S, Ross SA, Waugh DJ, Gaivin RJ, Morilak D, et al. Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: multiple system atrophy. Nat Med 2000;6:1388-94.
- Zuscik MJ, Chalothorn D, Hellard D, Deighan C, McGee A, Daly CJ, et al. Hypotension, autonomic failure, and cardiac hypertrophy in transgenic mice overexpressing the alpha 1B-adrenergic receptor. J Biol Chem 2001;276:13738-43.
- Ross SA, Rorabaugh BR, Chalothorn D, Yun J, Gonzalez-Cabrera PJ, McCune DF, et al. The alpha(1B)-adrenergic receptor decreases the inotropic response in the mouse Langendorff heart model. Cardiovasc Res 2003;60:598-607.
- Hague C, Bernstein LS, Ramineni S, Chen Z, Minneman KP, Hepler JR. Selective inhibition of alpha1A-adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J Biol Chem 2005;280:27289-95.
- Xu Q, Zhang T, Han QD, Zhang YY. Binding between alpha 1A-adrenergic receptor and segment of bone morphogenetic protein-1 in human embryonic cell 293. Acta Physiol Sin 2003;55:692-8. Chinese..
- Pupo AS, Minneman KP. Interaction of neuronal nitric oxide synthase with alpha1-adrenergic receptor subtypes in transfected HEK-293 cells. BMC Pharmacol 2002;2:17.
- Zhang T, Xu Q, Chen FR, Han QD, Zhang YY. Yeast two-hybrid screening for proteins that interact with alpha1-adrenergic receptors. Acta Pharmacol Sin 2004;25:1471-8.
- Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, et al. Spinophilin regulates Ca(2+) signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol 2005;7:405-11.
- Xu Z, Hirasawa A, Shinoura H, Tsujimoto G. Interaction of the alpha(1B)-adrenergic receptor with gC1q-R, a multifunctional protein. J Biol Chem 1999;274:21149-54.
- Diviani D, Lattion AL, Abuin L, Staub O, Cotecchia S. The AP2 complex directly interacts with the alpha-1b-adrenergic receptor and plays a role in receptor endocytosis. J Biol Chem 2003;278:19331-40.
- Pupo AS, Minneman KP. Specific interactions between gC1qR and alpha1-adrenoceptor subtypes. J Recept Signal Transduct Res 2003;23:185-95.
