Radioimmunotherapy of carcinoma of colon with [131I]-labeled recombinant chimeric monoclonal antibodies to carcinoembryonic antigen
Introduction
Human colonic carcinoma is one of the most common cancers. The 5-year survival rate of patients with chemotherapy is zero. More than half of the patients with this tumor experience metastasis or reoccurrence after treatment. The liver is the most common metastasis foci[1]. Radiolabeled MoAbs offer the prospect of a localized, highly targeted radiation treatment for these cancers. The range of action for radionuclides is defined predominantly by the nature of the particle and energy of the emission. One of the earliest radioisotopes to be coupled to antibodies for therapeutic purposes was Iodine 131 (131I). Its high-energy α particles can penetrate approximately three tumor cells, so it can be effective even when only deposited near the tumor cells and has minor toxicology to normal cells[2]. There are several antibodies for a variety of human tumors that have been used to localize human tumors in xenograft models as well as in patients. Several of these antigens have served as targets for testing whether MoAbs as conjugates with radionuclides can act as selective therapeutic agents. For example, antibodies directed against CEA, α-fetoprotein, ferritin, melanoma, and epithelial-specific antibody have been radiolabeled with 131I and used in the treatment of human cancers[3,4].
In histological classifications, colon cancers are over 90% adenocarcinoma. CEA can be observed in either the cancer cell surface or patients blood serum in this type of tumor[5]. Until recently, three products have been approved worldwide for the treatment of tumors in patients: Bexxar, Zevalin and ChTNT. The antibody used in this experiment is a new product awaiting permission for clinical trial, provided by Beijing Second Pharmaceutical Co, Ltd (Beijing). We undertook this study to determine the antitumor effect of the [131I]-labeled anti-CEA MoAbs and its distribution in nude mice bearing xenografts.
Materials and methods
Mice Athymic nude female BABL/c nu/nu mice, 4–6 weeks old, were obtained from the Institute of Laboratory Animals, Chinese Medical Science Academy. Mice were kept under SPF conditions and were fed with a diet of sterile mice chow and water. Animals were given 10% Lugol’s (5% Iodine and 10% KI) water from 2 d before the start of the experiment beginning until the experiment was completed.
Cell lines Three colonic carcinoma derivative cell lines were used: LS180 (ATCC N
[131I]-labeled anti-CEA MoAbs [131I]-labeled anti-CEA humanized chimeric recombinant MoAbs ([131I]-labeled-rch24) were supplied by Beijing SaiKe Pharmaceutical. Radioactivity was 5 mCi/mg. Radiochemical purity was more than 98.5%.
Establishing colon tumors in nude mice The three tumor cells were harvested and suspended in sterile PBS at a concentration of 25×106 cells/mL. Cell viability was determined by trypan blue dye exclusion. Cells (5×106) in sterile PBS were inoculated subcutaneously into the flank of nude mice[7]. Tumors became apparent in 8–10 d.
Radiolabeled antibody treatment of tumors Mice bearing tumors were randomly divided into groups outlined in Table 1. Mice were administered i.v. in the tail vein. Antibodies were given 2 times with the interval of 10 d. The positive chemotherapy drug (5-FU) was given 2 weeks, 6 times a week.

Full table
Radiolabeled antibody effect The tumor growth rate was determined by measuring the length (a) and width (b) (mm) of each tumor using a caliper. Tumor volume=a×b2/2. The relative tumor volume (RTV), RTV=Vt/Vo. Vo is the tumor volume when the experiment started. Vt is the measured tumor volume at different experiment time. The relative tumor growth rate was calculated by % of T/C=TRTV (treated group)/CRTV (control group)×100%. The effective criterion is T/C (%) above or equal to 60%. Tumor growth inhibition rate was calculated by S%=(mean weight of treated group-mean weight of control group)/(mean weight of control group)×100%[8].
To evaluate peripheral plasma CEA levels, mice in each group were bled from the eye using heparinized capillary tubes. The plasma CEA level was determined by ELISA (Hoffmann-La Roche Ltd).
Radiolabeled antibody biodistribution Two animals from each group were bled, killed, and dissected at 24 h, 48 h, or 96 h after treatment, respectively. Tissues and organs were immediately dissected, rinsed with saline, blotted dry, and placed in plastic tubes and weighed. The radioactivity of each sample of blood, liver, heart, lung, kidney, and tumor tissue was measured using a well-type gamma counter. From the data, [131I]-labeled anti-CEA MoAbs biodistributions (%ID/g) were calculated: %ID/g=(tissue or organ cpm)/(total injected cpm)/ (tissue or organ weight).
Statistical analysis Differences among the groups were tested using a one-way ANOVA. Results are given as mean±SD unless indicated otherwise.
Results
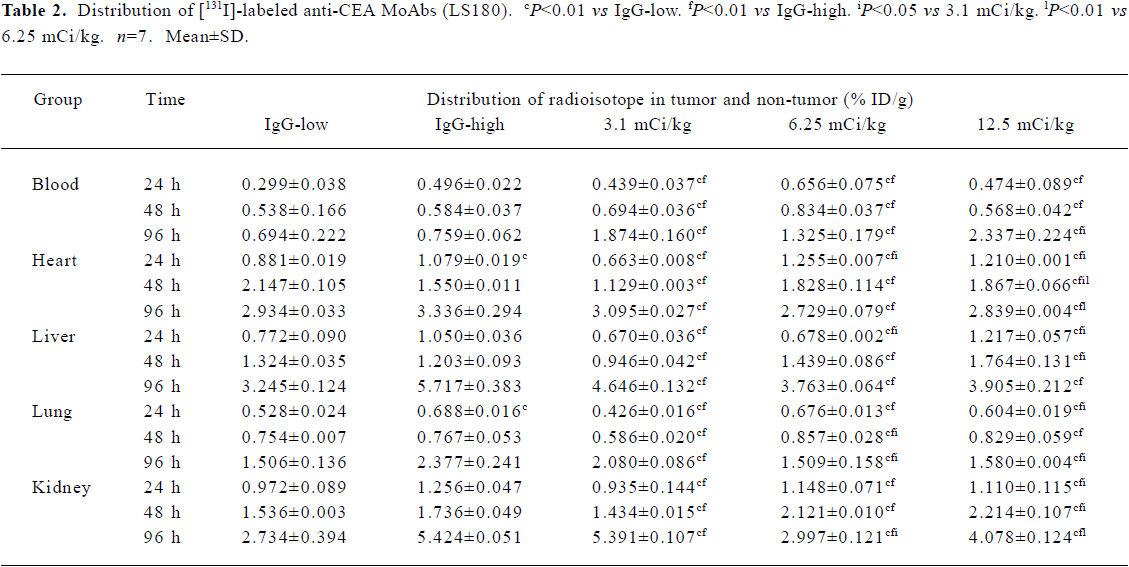
Distribution studies Tables 2, 3 and 4 summarize the tumor/non-tumor ratios found with either [131I]-labeled anti-CEA MoAbs or [131I]-labeled-IgG in mice with tumors. The results confirmed the tumor-specific targeting and retention of [131I]-labeled anti-CEA MoAbs in tumor tissues in contrast to [131I]-labeled-IgG. While the percentage of injected dose per gram (%ID/g) in the normal tissues continued to decrease over time for both [131I]-labeled anti-CEA MoAbs and [131I]-labeled-IgG, the percentage of [131I]-labeled anti-CEA MoAbs increased in the tumor between d 1 and 4. This caused the T/NT ratios continue to increase in this period. T/NT ratios for [131I]-labeled anti-CEA MoAbs were 2–2.5 times higher than [131I]-labeled-IgG on d 1 and continued to increase so that T/NT ratios were 10–20 times higher than [131I]-labeled-IgG by day 4.
Full table

Full table

Full table
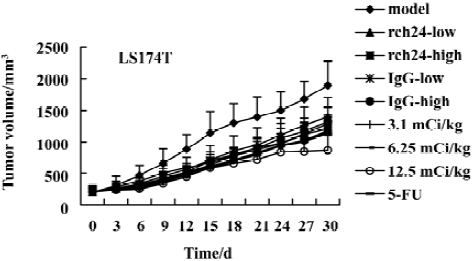
Inhibition of tumor growth The biological effect of [131I]-labeled anti-CEA MoAbs in mice bearing three tumor types was assessed. The tumor growth curves are summarized in Figures 1, 2 and 3. The volume of both [131I]-labeled anti-CEA MoAbs groups was less than the control group. As the administrative dosage increased, the tumor volume increment rate became slow or was not obvious.
The relative tumor growth rate of three tumor types was calculated. The growth of tumors were inhibited significantly at the dosage groups of 3.1 mCi/kg, 6.25 mCi/kg, and 12.5 mCi/kg in nude mice bearing LS180 or LS174T (T/C%<60%). For SW1116, only the 6.25 mCi/kg and 12.5 mCi/kg dosages were effective. With the increasing dosage, more obvious inhibition of the tumor growth was observed.
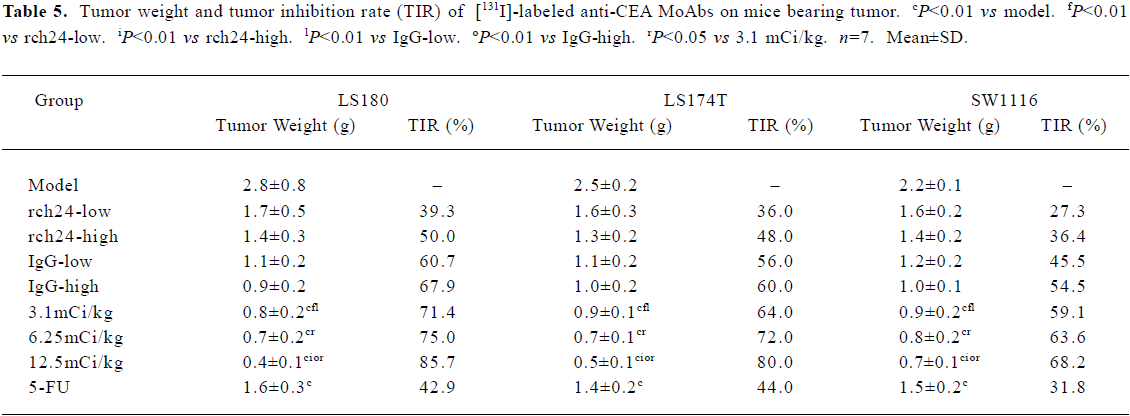
Tumor weight and tumor growth inhibition rate (TIR) were calculated. The data is shown in Table 5. The tumor weights of three dosage [131I]-labeled anti-CEA MoAbs groups were all less than that of the control. With the increase in dosage, the tumor growth inhibition rate was more obvious. The tumor growth inhibition rate of the 3.1 mCi/kg CEA MoAbs group (LS180, LS174T, SW1116) was 47.8%–64.0%. This was 69.6%–78.6% in the 6.25 mCi/kg CEA MoAbs group, and 81.8%–86.2% in the 12.5 mCi/kg [131I]-labeled anti-CEA MoAbs group.
Full table
Plasma CEA level The plasma CEA level is shown in Table 6. Three groups’ CEA levels were lower than the control group. This shows a relationship between the CEA level and dosage. Compared with the “nude” antibody and [131I]-labeled-IgG, [131I]-labeled anti-CEA MoAbs was more effective in lowering the CEA level.

Full table
Discussion
A new approach in radiation therapy for cancer involves the use of radiolabeled MoAbs raised against tumor-associated antigens[9]. The approach adopted in this study was the use of [131I]-labeled anti-CEA MoAbs at different doses to produce tumor growth inhibition in groups of athymic nude mice bearing human colon adenocarcinoma xenografts. The two principal objectives of this study were to examine the biodistribution and antitumor activity of the [131I]-labeled anti-CEA MoAbs.
Our data show that [131I]-labeled anti-CEA MoAbs at different dosages can significantly inhibit the growth rate of tumors (LS180, LS174T, SW1116) in a dose-dependent manner. We are encouraged by the finding that the destruction of tumors was apparent in approximately 50% of tumors in the animals given 3.1 mCi/kg of radiolabeled rch24 antibody. This suggests that we may be able to use a low dosage to produce slight toxicity.
With one exception, most therapeutic studies with [131I]-labeled antibodies in experimental animals have failed to inhibit completely the growth of well-established tumors[10–12]. However, Cheung et al were able to ablate 0.5–2.0 cm3 neuroblastoma xenografts in nude mice with a single injection of 1 mCi of [131I]-labeled 3F8 MoAbs[13]. Whether these results are a result of a property of the antibody, radiosensitivity of the tumor or some other factor, is unclear, but all current experimental evidence indicates that radiolabeled antibodies can be effectively used to inhibit tumor growth.
In this report we examined the distribution of [131I]-labeled anti-CEA MoAbs. Targeting was observed 24 h after the drug was administered. It was more obvious 96 h after administration. The blood and liver have the main uptake and the kidney has a low uptake. Toxicity was measured by the change in bodyweight and by determination of the total peripheral white blood cells (WBC). There was no significant difference in the bodyweight and peripheral WBC counts between the treated groups and model control (data not shown).
Because [131I] is not an as effective radionuclide as other isotopes, other radioconjugates are being pursued[14,15]. One of the best candidates for convenient coupling to antibodies is Yttrium-90 [90Y]. But there are difficulties in the application. These include high uptake in normal tissue, especially the liver, and problems associated with obtaining high specific activity [90Y]. In addition, [90Y] are known to concentrate in the bone[16]. This may cause severe problems. Each radionuclide antibody tumor system has advantages and disadvantages, but [131I] label is the most promising method at present.
Overall, the results of the present study indicate that tumor growth inhibition using radiolabeled antibodies can be confirmed. Using selectively localizing antitumor antibodies conjugated with suitably cytotoxic radionuclides may provide a useful new approach to the treatment of disseminated cancers.
References
- Debinski W, Karlsson B, Lindholm L, Siegall CB, Willingham MC, FitzGerald D, et al. Monoclonal antibody C242-Pseudomonas exotoxin A. A specific and potent immunotoxin with antitumor activity on a human colon cancer xenograft in nude mice. J Clin Invest 1992;90:405-11.
- Ditzel HJ, Garrigues U, Andersen CB, Larsen MK, Garrigues HJ, Svejgaard A, et al. Modified cytokeratins expressed on the surface of carcinoma cells undergo endocytosis upon binding of human monoclonal antibody and its recombinant Fab fragment. Proc Natl Acad Sci USA 1997;94:8110-5.
- Philben VJ, Jakowatz JG, Beatty BG, Vlahos WG, Paxton RJ, Williams LE, et al. The effect of tumor CEA content and tumor size on tissue uptake of indium 111-labeled anti-CEA monoclonal antibody. Cancer 1986;57:571-6.
- Buchegger F, Pfister C, Fournier K, Prevel F, Schreyer M, Carrel S, et al. Ablation of human colon carcinoma in nude mice by 131I-labeled monoclonal anti-carcinoembryonic antigen antibody F(ab’)2 fragments. J Clin Invest 1989;83:1449-56.
- Dohlsten M, Hedlund G, Akerblom E, Lando PA, Kalland T. Monoclonal antibody-targeted superantigens: a different class of anti-tumor agents. Proc Natl Acad Sci USA 1991;88:9287-91.
- Han JS, Nair PP. Flow cytometric identification of cell surface markers on cultured human colonic cell lines using monoclonal antibodies. Cancer 1995;76:195-200.
- Xu X, Clarke P, Szalai G, Shively JE, Williams LE, Shyr Y, et al. Targeting and therapy of carcinoembryonic antigen-expressing tumors in transgenic mice with an antibody-interleukin 2 fusion protein. Cancer Res 2000;60:4475-84.
- Buchegger F, Mach JP, Pèlegrin A, Gillet M, Vogel CA, Buclin T, et al. Radiolabeled chimeric anti-CEA monoclonal antibody compared with the original mouse monoclonal antibody for surgically treated colorectal carcinoma. J Nucl Med 1995;36:420-9.
- Hajjar G, Sharkey RM, Burton J, Zhang CH, Yeldell D, Matthies A, et al. Phase I radioimmunotherapy trial with iodine-131-labeled humanized MN-14 anti-carcinoembryonic antigen monoclonal antibody in patients with metastatic gastrointestinal and colorectal cancer. Clin Colorectal Cancer 2002;2:31-42.
- Zhu H, Baxter LT, Jain RK. Potential and limitations of radio- immunodetection and radioimmunotherapy with monoclonal antibodies. J Nucl Med 1997;38:731-41.
- Ychou M, Pèlegrin A, Faurous P, Robert B, Saccavini JC, Guerreau D, et al. Phase I/II radio-immunotherapy study with iodine-131-labeled anti-CEA monoclonal antibody F6 F(ab’)2 in patients with non-resectable liver metastases from colorectal cancer. Int J Cancer 1998;75:615-9.
- Cheung NK, Landmeier B, Neely J, Nelson AD, Abramowsky C, Ellery S, et al. Complete tumor ablation with iodine 131-radiolabeled disialoganglioside GD2-specific monoclonal antibody against human neuroblastoma xenografted in nude mice. J Natl Cancer Inst 1986;77:739-45.
- Denardo SJ, O’Grady LF, Richman CM, Goldstein DS, O’Donnell RT, Denardo DA, et al. Radioimmunotherapy for advanced breast cancer using I-131-ChL6 antibody. Anticancer Res 1997;17:1745-51.
- Sharkey RM, Brenner A, Burton J, Hajjar G, Toder SP, Alavi A, et al. Radioimmunotherapy of non-Hodgkin’s lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-Epratuzumab): do tumor targeting and dosimetry predict therapeutic response? J Nucl Med 2003;44:2000-18.
- Wong JY, Shibata S, Williams LE, Kwok CS, Liu A, Chu DZ, et al. A Phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer. Clin Cancer Res 2003;9:5842-52.