Anti-implantation effect of droloxifene in rats and its relationship with anti-estrogenic activity1
Introduction
Droloxifene, a derivative of the triphenylethylene drug tamoxifen, is a novel selective estrogen receptor modulator (SERM)[1]. Its higher affinity to the estrogen receptor, higher anti-estrogenic to estrogenic ratio, more effective inhibition of cell growth and division in estrogen receptor-positive cell lines, and lower toxicity give it theoretical advantages over tamoxifen in the treatment of human breast cancer[2]. Droloxifene may also be a potentially useful agent for the treatment of postmenopausal osteoporosis because it can prevent estrogen deficiency-induced bone loss without causing uterine hypertrophy[3]. Droloxifene may have an effect on bone and breast tissue because it induces apoptosis[4]. The corpus luteum is an ovarian tissue that synthesizes and secretes progesterone, which plays a key role in the establishment and maintenance of pregnancy in mammals. Abnormal regression of the corpus luteum will disturb or even terminate both the implantation process and early pregnancy. Apoptosis is involved in the regression of the corpus luteum in many species[5]. Therefore, better understanding the compounds that induce the apoptosis of luteal cells may contribute to the development of new anti-implantation agents. Our laboratory was the first to report that droloxifene induceed the apoptosis of rat luteal cells in vitro and the pre-implantation luteal cells in pregnant rats[6–8]. Moreover, droloxifene facilitates the apoptosis of luteal cells and shortens the period of pseudopregnancy in pseudopregnant rats[9]. These results suggest that droloxifene induces the regression of the corpus luteum and has potential anti-implantation effects. Exact equilibrium of estrogen and progesterone is essential for implantation, and any disturbance in the effects of these hormones can cause infertility[10]. As a novel selective estrogen receptor modulator with greater anti-estrogenic effects, droloxifene seems to interfere with the effect of estrogen and cause anti-implantation effects. However, the anti-implantation effect of droloxifene has not been evaluated and reported on. Therefore, in the present study, the anti-implantation effect of droloxifene was evaluated and the relationship between the anti-estrogenic activity of droloxifene and its anti-implantation effect was analyzed in rats.
Materials and methods
Drugs and reagents The droloxifene was synthesized by Prof Peng XIA (Department of Organic Chemistry, College of Pharmacy, Fudan University, Shanghai) and was suspended in 1% sodium carboxymethylcellulose (CMC). Estradiol (E2) was purchased from the Shanghai 9th Pharmaceutical Factory (Shanghai, China) and was suspended in corn oil. Serum estrogen and progesterone radioimmunoassay (RIA) kits were obtained from DEPU Ltd (Tianjin, China).
Animals and treatment Sprague-Dawley rats (body weight: female, 220–250 g an male 300–350 g, SIPPR/BK LtdShanghai) were kept in a temperature-controlled (24–26 °C) and light-regulated (12 h light, 12 h dark) room, and were given ad libitum access to standard chow and water. The female animals were cohabited with male animals at a ratio of 2:1. The day that sperm were found in the vaginal smear was designated as d 1 of pregnancy.
Evaluation of anti-implantation efficacy Confirmed pregnant female rats were randomly assigned into different groups and were treated with droloxifene (ig), estradiol (E2, sc), or 1% CMC (as a control, ig). The doses and treatment times of the different experiments are shown in the results section. At autopsy on d 9, the number of animals with implantation sites in each group was recorded. The Bliss method was used to calculate the ED95, ED90, and ED50 of the anti-implantation effect of droloxifene.
Assay of the serum levels of estrogen and progesterone The pregnant rats were treated orally with 2.5 mg/kg droloxifene or 1% CMC at 22:00 PM on d 4. Blood samples of 0.5 mL were obtained from the tail veins of pregnant rats at 10:00 AM on d 1, d 2, d 3, d 4, d 5 and d 6, and at 22:00 PM on d 4. The serum levels of estrogen and progesterone were measured by RIA according to the manufacturer’s inst- ructions.
Statistical analysis Differences in pregnancy rates between the groups were tested by using the χ2 test. Serum levels of estrogen and progesterone are expressed as mean±SD and Student’s t-test was used to calculate signi- ficance.
Results
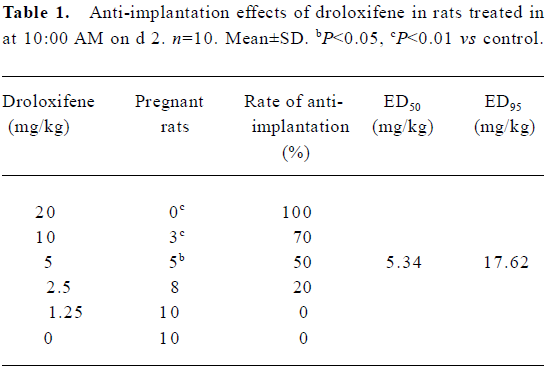
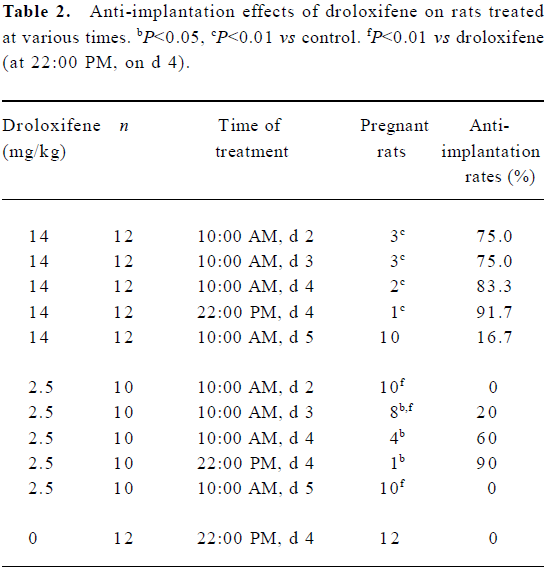
Optimal administration time for the anti-implantation effect of droloxifene Pregnant rats were treated orally with droloxifene with doses ranging from 1.25 to 20.0 mg/kg at 10:00 AM on d 2. Within the treatment time, droloxifene had an anti-implantation effect in rats (ED95=17.62 mg/kg and ED50=5.34 mg/kg; Table 1). To determine the optimal administration time for the anti-implantation effect of droloxifene, pregnant rats were treated orally with droloxifene at either a high dose (14 mg/kg) or a low dose (2.5 mg/kg) at 10:00 AM on d 2, d 3, d 4, or d 5, or at 22:00 PM on d 4. Although the differences in the anti-implantation rates in different groups were not significant for the 14 mg/kg groups, the anti-implantation rates of rats treated with droloxifene at 22:00 PM on d 4 were the highest in the two dose groups (Table 2). These results suggest that droloxifene has anti-implantation effects in rats, and that the optimal administration time is at 22:00 PM on d 4.
Full table
Full table
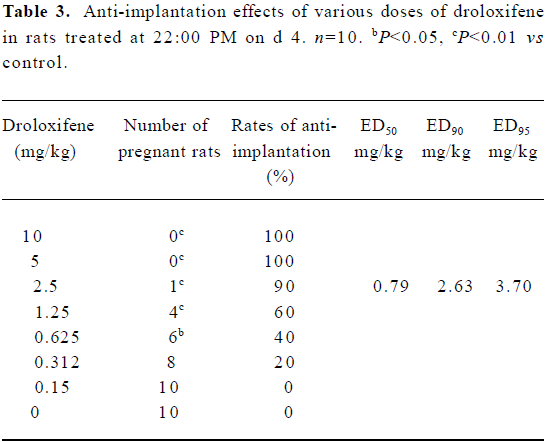
ED95, ED90, and ED50 for the anti-implantation effect of droloxifene Pregnant rats were treated orally with drol- oxifene at various doses (10, 5.0, 2.5, 1.25, 0.62, 0.31, or 0.15 mg/kg) at 22:00 PM on d 4. The anti-implantation rates of the droloxifene groups (0.62–10 mg/kg) were higher than that observed in the control group (P<0.01; Table 3). There was a dose-dependent relationship between the anti-implantation rates and droloxifene doses from 0.15 mg/kg to 5.0 mg/kg. The values of ED95, ED90 and ED50 were 3.70 mg/kg, 2.63 mg/kg and 0.79 mg/kg, respectively.
Full table
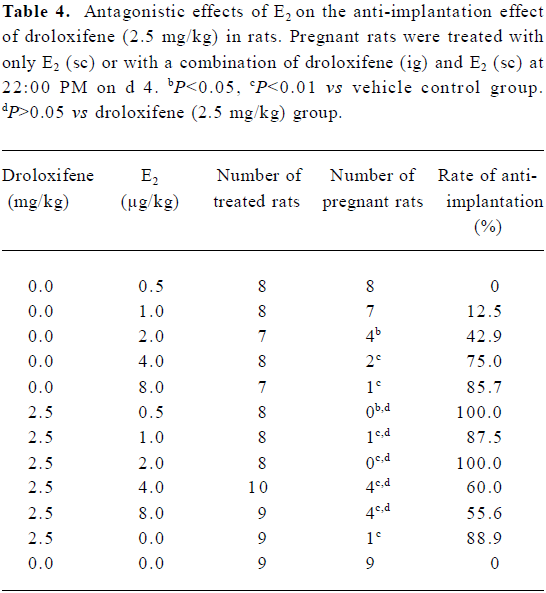
Antagonistic effect of external E2 on the anti-implantation effect of droloxifene To investigate the antagonistic effect of external E2 on the anti-implantation effect of droloxifene, the anti-implantation effect of external E2 only was first evaluated. When rats were treated at 22:00 PM on d 4 with external E2 at doses of 2.0 µg/kg or 8.0 µg/kg (sc), significant anti-implantation effects were observed (P<0.05), whereas at doses of 0.5 µg/kg or 1.0 µg/kg, there was no anti-implantation effect. For rats treated at 22:00 PM on d 4 with 2.5 mg/kg droloxifene alone or 2.5 mg/kg droloxifene combined with various doses of E2, there was no difference in implantation rates, although E2 at higher doses (2.0 or 8.0 µg/kg) reduced the anti-implantation rates (Table 4).
Full table
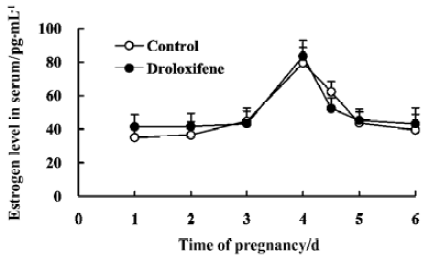
Effect of droloxifene on the serum level of estrogen during early pregnancy Pregnant rats were treated orally with 2.5 mg/kg droloxifene or 1% CMC at 22:00 PM on d 4. The rate of implantation was 100% and 0% in the control and droloxifene groups, respectively. In the control group, the serum level of estrogen remained at low levels from d 1 to d 3, began to rise on d 3, reached the maximum at 10:00 AM on d 4, then declined sharply, such that the levels on d 5 and d 6 were similar to those on d 3. The serum estrogen levels in the droloxifene group between d 1 and d 6 were not significantly different from those in the control group (Figure 1). These results indicate that there was a surge of estrogen in the pregnant rats at 10:00 AM on d 4, and that treatment with droloxifene at 22:00 AM on d 4 had no effect on the level of estrogen; however, a significant anti-implantation effect was induced. Therefore, the anti-implantation effect of droloxifene in rats appears not to be due to antagonism of a surge in the secretion of nidatory estrogen.
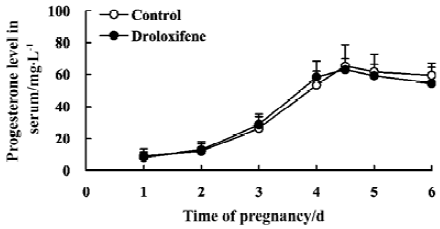
Effect of droloxifene on serum levels of progesterone during early pregnancy in rats Pregnant rats were treated orally with 2.5 mg/kg droloxifene or 1% CMC at 22:00 PM on d 4. The rates of implantation were 100% and 0% in the control and droloxifene groups, respectively. In the control groups, the serum levels of progesterone rose from 10:00 AM on d1 to 22:00 PM on d 4 and remained at high levels until d 6. In groups treated with droloxifene at 22:00 PM on d 4, the levels of progesterone were similar to that of controls (Figure 2). These results indicate that treatment with droloxifene at 22:00 PM on d 4 had no effect on the level of progesterone in early pregnancy.
Discussion
It is well established that an exact equilibrium of estrogen and progesterone is indispensable for implantation in rats[10]. A nidatory c surge that occurs on d 4 is essential for the sensitization of the uterus to induce decidual cell reaction, the most specific function of the progestational endom- etrium[11,12]. As a novel selective estrogen receptor modulator with considerable anti-estrogenic effects, droloxifene might disturb the hormonal effects and cause an anti-implantation effect. The present study found that droloxifene had anti-implantation effects in rats (Tables 1–3) and that 22:00 PM on d 4 was the optimal oral administration time. At this time there was a good dose-effect relationship between the anti-implantation rates and droloxifene doses from 0.31 mg/kg to 5.0 mg/kg. The ED95, ED90 and ED50 of droloxifene were 3.70 mg/kg, 2.63 mg/kg, and 0.79 mg/kg, respectively.
We found that the serum levels of estrogen in pregnant rats reached a peak at 10:00 AM on d 4, which indicates that the nidatory estrogen surge before implantation occurs at approximately this time. However, the optimal oral administration time of droloxifene for anti-implantation effects was at 22:00 PM on d 4, 12 h later than the nidatory estrogen surge. Therefore, we propose that the anti-implantation effect of droloxifene is not caused by its interfering with the nidatory estrogen surge via its anti-estrogenic effect. The effects of droloxifene are different from those of tamoxifen, a triphenylethyl compound, which antagonizes the nidatory estrogen surge[13,14].
In order to further clarify the relationship between the anti-implantation effect and the anti-estrogenic activity of droloxifene, the antagonistic effect of external E2 on the anti-implantation effect of droloxifene was observed in rats. At first, the anti-implantation effect of external estrogen (0.5–8.0 µg/kg, sc) was examined after administration at 22:00 PM on d 4. We found that E2 at doses of 0.5–1.0 µg/kg produced no anti-implantation effect (Table 4), and had no antagonistic effect on the anti-implantation effect of droloxifene (P>0.05; Table 4). When droloxifene was combined with E2 at higher doses (4.0 or 8.0 µg/kg), the anti-implantation effect of droloxifene was reduced, but the difference was not significant according to the χ2 test. Therefore, it seems that the anti-implantation effect of droloxifene may be not related to its anti-estrogenic activity, especially at physiological doses.
Because an exact equilibrium of estrogen and progesterone is essential for implantation, and any disturbance in the effects of these hormones can cause infertility, we investigated whether droloxifene inhibited implantation by affecting the serum levels of estrogen and progesterone. We found that droloxifene had no effect on the serum estrogen and progesterone levels in early pregnancy when treated at 22:00 PM on d 4. However, in our previous study, we found that apoptosis of luteal cells and decreases in serum progesterone levels were induced by treatment with droloxifene at a dose of 20 mg/kg on d 2 in pregnant rats[8]. The differences between the two experiments can be explained by the different doses and administration times. In addition, the period of observation was too short in the present study.
In conclusion, droloxifene can inhibit implantation in rats and the optimal oral administration time is 22:00 PM on d 4. ED90 was 2.63 mg/kg. The anti-implantation effect of droloxifene is not related to its antiestrogenic activity, or an antagonistic effect on the nidatory estrogen surge. The direct inhibition of endometrial receptivity to blastocyst signal(s) and the apoptosis of luteal cells might be involved in the anti-implantation mechanism of droloxifene. This charac-teristic may make droloxifene useful in developing new contraceptives.
Acknowledgment
We are grateful to Prof Zhi-ping GU for valuable discussions throughout this study and helpful comments on the manuscript.
References
- Eppenberger U, Wosikowski K, Kung W. Pharmacologic and biologic properties of droloxifene, a new antiestrogen. Am J Clin Oncol 1991;14:S5-14.
- Hasmann M, Rattel B, Loser R. Preclinical data for droloxifene. Cancer Lett 1994;84:101-6.
- Ke HZ, Chen HK, Qi H, Pirie CM, Simmons HA, Ma YF, et al. Effects of droloxifene on prevention of cancellous bone loss and bone turnover in the axial skeleton of aged, ovariectomized rats. Bone 1995;17:491-6.
- Grasser WA, Pan LC, Thompson DD, Paralkar VM. Common mechanism for the estrogen agonist and antagonist activities of droloxifene. J Cell Biochem 1997;65:159-71.
- Palumbo A, Yeh J. Apoptosis as a basic mechanism in the ovarian cycle: follicular atresia and luteal regression. J Soc Gynecol Investig 1995;2:565-73.
- Leng Y, Yang B, Cao L, Gu ZP. Effects of anordrin, droloxifene, nomegestrol, and mifepristone on cultured rat luteal cell apoptosis. Acta Pharmacol Sin 1999;20:400-4.
- Leng Y, Gu ZP, Cao L. Apoptosis induced by droloxifene and c-myc, bax and bcl-2 mRNA expression in cultured luteal cells of rats. Eur J Pharmacol 2000;409:123-31.
- Leng Y, Gu ZP, Cao L. Apoptosis induced by droloxifene and C-myc, Bax, Bcl-2 protein expression in corpus luteum of pregnant rats. Acta Pharmacol Sin 2001;22:327-34.
- Leng Y, Feng Y, Cao L, Gu ZP. Effects of droloxifene on apoptosis and Bax, Bcl-2 protein expression of luteal cells in pseudopregnant rats. Acta Pharmacol Sin 2001;22:155-62.
- Kodaman PH, Taylor HS. Hormonal regulation of implantation. Obstet Gynecol Clin North Am 2004;31:745-66.
- Finn CA. Oestrogen and decidual cell reaction of implantation in mice. J Endocrinol 1965;32:223-9.
- Liu Y, Jia XC. Changes of oestrogen and its receptor levels during implantation period in pregnant rats. Acta Physiol Sin 1981;33:44-50.
- Watson J, Anderson FB, Alam M, O’Grady JE, Heald PJ. Plasma hormones and pituitary luteinizing hormone in the rat during the early stages of pregnancy and after post-coital treatment with tamoxifen (ICI 46,474). J Endocrinol 1975;65:7-17.
- Bloxham PA, Pugh DM, Sharma SC. Abolition of the pre-implantation surge of plasma oestrogens in mice with tamoxifen. Irc Med Sci Reprod Obstet Gynecol 1977;5:432.
