Comparative study on pharmacological effects of DM-phencynonate hydrochloride and its optical isomers1
Introduction
In order to develop more potent drugs for muscarinic intervention in disorders of the central and peripheral nervous system, a series of heterocyclic compounds including esters, alkanes, and ether derivatives were designed and synthesized in our institute. One of the compounds, CPG, has been developed as a novel anti-motion-sickness drug with higher efficacy and lower central inhibitory side effects than diphenidol HCl and scopolamine HBr, and is used clinically[1–3]. (±)DMCPG, which has one chiral center and two enantiomers [R(–) and S(+)DMCPG] was the active dominant N-demethyl metabolite of CPG. We believe that this is an important way of looking for active compounds from the metabolites to discover new medicines. We synthesized (±)DMCPG and its two enantiomers (Figure 1). To avoid adverse effects and to optimize the therapeutic value of enantiomeric drugs, it is necessary to establish the effectiveness of isomers of the chiral drug, and to detect the presence of the enantiomer with lower therapeutic activity and undesirable adverse effects. For this purpose, we investigated the binding characteristics of these new chiral compounds by using a radioligand receptor-binding assay with muscarinic receptors from rat brain, and compared its pharmacological effects on muscarinic receptors by means of in vitro and in vivo assays.
Materials and methods
Animals The experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, from the National Research Council (1996), under the approved protocols. Animals used in the present study were as follows: Kunming species mice weighing 18–22 g [grade II, certificate N
Compounds 3H-quinuclindinyl benzilate ([3H]QNB; 43.3 Ci/mmol) was purchased from Amersham (Uppsala, Sweden; TRK604). (±)DMCPG and its isomers were synthesized at our institute. The (±)DMCPG comprised equal proportions of R(–) and S (+)DMCPG. Atropine, pentobarbital, oxotremorine and carbachol were purchased from Sigma (St Louis, MO, USA).
Binding assays on rat cerebral cortex homogenate Male or female Wistar rats were killed by decapitation. The cerebral cortex was immediately removed and processed as described by Yamamura and Snyder[4].The protein concentration was determined by using the method of Lowry et al[5]. The samples of homogenate (each containing 50 mg of protein) were incubated for 30 min at 37 ºC in 0.5 mL of assay buffer containing 6 nmol/L [3H]QNB and various concentrations of drugs. In saturation binding assays, the homogenate was incubated as indicated above, in the presence of [3H]QNB (0.25–20 nmol/L). Non-specific binding was defined as binding in presence of atropine (1 µmol/L). Each sample was filtered through GF/C glass fibers with vacuum. The filters were rinsed 3 times with 3 mL cold buffer, and placed in scintillation vials containing 3 mL of scintillation fluid. Radioactivity trapped on the filters was determined by liquid scintillation spectrometry at approximately 40%–50% efficiency.
Carbachol-induced contraction Male guinea pigs were killed by cervical dislocation. The organs required were set up rapidly under 1 g of tension in 20 mL organ baths containing physiological salt solution (PSS), which was kept at 37 ºC and aerated with 5% CO2 and 95% O2. Two-centimeter-long portions of terminal ileum were taken at about 5 cm from the ileum–cecum junction and mounted in PSS at 37 ºC. The composition of PSS was as follows (mmol/L): NaCl 118, NaHCO3 23.8, KCl 4.7, MgSO4·7H2O 1.18, KH2PO4 1.18, CaCl2 2.52, glucose 11.7. Tension changes were recorded isotonically. Tissues were equilibrated for 90 min before the experiments were conducted.
The carbachol-induced ileum contraction was obtained by incubation with carbachol (10-5 mol/L) until the concentration had reached a plateau. Then, the ileum strips were washed several times with PSS until the tension of the strips relaxed to the baseline level. Following washing, the strips were incubated with different concentrations of R(–), S(+), or (±)DMCPG for 10–15 min. After incubation, the maximum contraction induced by carbachol (10-5 mol/L) was observed again in the presence of increased concentrations of different antagonists. The IC50 values for the carbachol-induced contractions in the presence of the antagonists were obtained to assess the pharmacological potency of (±)DMCPG and its optical isomers.
Effect on sub-threshold hypnotic dose of sodium pentobarbital induced-sleep Four dosage groups were used for each drug and each group consisted of 10 mice of each sex. Mice were pretreated with (±)DMCPG and its optical isomers intraperitoneally (ip), then after 15 min, a sub-threshold hypnotic dose of sodium pentobarbital (30 mg/kg) was given ip. The loss of righting reflex was used as a measure of the central inhibitory effect of drugs. The ED50 values of these three drugs were estimated to compare the central inhibitory effect of the indicated agents.
Inhibition of oxotremorine-induced salivation Kunming mice were assigned randomly into 4 groups for each drug. Each group consisted of 10 mice of each sex. (±)DMCPG and its optical isomers were administered ip 15 min prior to oxotremorine (3 mg/kg) being injected subcutaneously (sc). ED50 values were calculated to evaluate the anti-secretive potencies of the compounds used.
Data analysis and statistics
Binding assays The IC50 values were obtained from at least three separate experiments performed in triplicate with 6-8 concentrations of drugs. Data were analyzed by curvilinear regression using the program ORIGIN 6.0. The inhibition constants (Ki) were calculated using the Cheng-Prusoff equation[6], Ki=IC50/(1+L/Kd), where L and Kd are the concentration and the equilibrium dissociation constant of [3H]QNB, respectively.
Functional assays In the carbachol-induced contraction experiments, the maximum contractile response (Emax) was obtained from the maximum stress, and the IC50 value was calculated from a semi-logarithmic plot of the percentage of the maximum response versus drug concentration. Data were computer analyzed by curvilinear regression using the program ORIGIN 6.0. Statistical analyses for comparisons among groups were performed using analysis of variance (ANOVA). P<0.05 was considered statistically significant. To evaluate the effect of (±)DMCPG and its optical isomers on anti-salivation induced by oxotremorine and sleeping induced by a sub-threshold hypnotic dose of sodium pentobarbital, ED50±95% CL (confidence limit) values were calculated and compared by weighted probit analysis. Data are shown as mean±SD.
Results
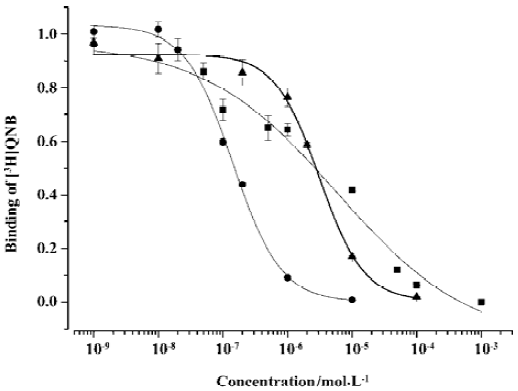
Competitive binding of (±)DMCPG and its optical isomers to rat central muscarinic acetylcholine receptors The Kd value for [3H]QNB binding to receptors was 6.66±0.95 nmol/L. The Bmax was 760±92 fmol/mg. The competitive binding potency of R(–)DMCPG for [3H]QNB corresponded to a Ki value of 763.75±7.31 nmol/L (n=4). An average Hill coefficient (nH) was 1.17±0.15. The affinity of R(–)DMCPG at central muscarinic acetylcholine receptors was higher than that of (±)DMCPG (Ki=3180±263 nmol/L, nH=0.42) and S(+) DMCPG (Ki=1699±260 nmol/L, nH=1.26). The isomer with R(–) configuration was more potent than the isomer with S(+) configuration and (±)DMCPG. The competition profiles of R(–)DMCPG and S(+)DMCPG to rat cortex muscarinic receptors were steep and adequately described by a one-site model, nH>1, which exhibited positive cooperative effects at muscarinic receptors. However, for (±)DMCPG, nH<1, which exhibited negative cooperative effects at muscarinic receptors, which is not consistent with a one-site binding model (Figure 2).
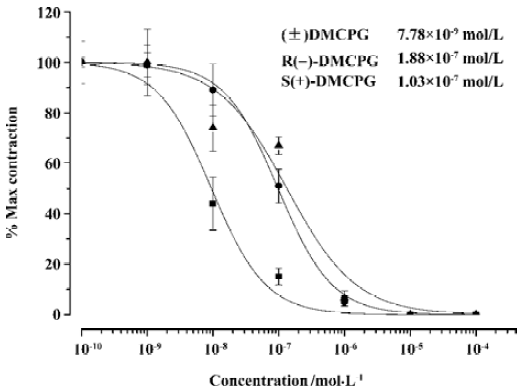
Effect of (±) DMCPG and its optical isomers on carbachol-induced contraction Carbachol (10-5 nmol/L) caused contractions in guinea pig ileum. The Emax values for the carbachol-induced contractions were 2.90±0.17 g (n=30). (±)DMCPG and its optical isomers (10-9–10-4 mol/L) significantly suppressed the carbachol-induced contractions (P<0.05; Figure 3). The IC50 values of (±) DMCPG, R(–)DMCPG and S(+)DMCPG were 7.78×10-9, 1.88×10-7, and 1.03×10-7 mol/L, respectively. The results revealed that (±)DMCPG was more potent in the anti-contraction of smooth muscle induced by carbachol than the other two configurations (P<0.05).
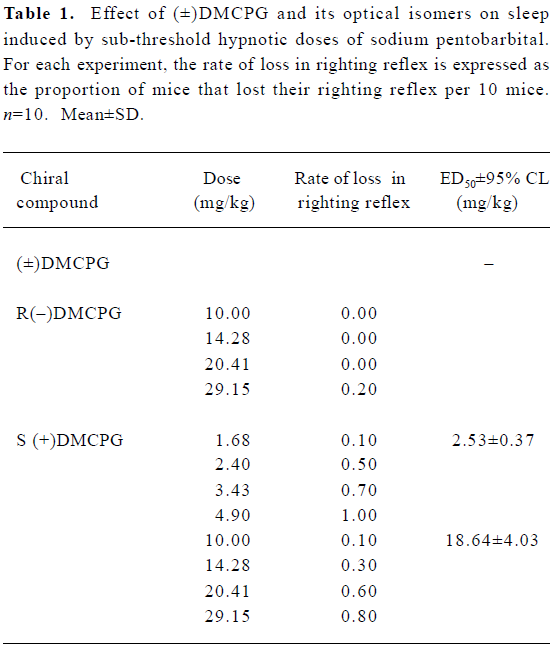
Potentiation of the effect of a sub-threshold hypnotic dose of sodium pentobarbital Pentobarbital (ip, 30 mg/kg) alone did not cause sleep in mice (n=50). However, after pretreatment with R(–)DMCPG (1.68–4.89 mg/kg) and S(+)DMCPG (10–29.15 mg/kg) at 15 min intervals, sedation effects induced by a sub-threshold hypnotic dose of sodium pentobarbital were enhanced in a dose-dependent manner (Table 1). The ED50 values ±95% confidence limits of R(–) and S(+)DMCPG were 2.53±0.37 and 18.65±4.03 mg/kg. Of the 10 mice pre-administered with (±)DMCPG at the highest dose (29.15 mg/kg), only 2 mice lost their righting reflex, which indicates that (±)DMCPG has a weak central depressant action. The order of central inhibition effects was R(–)DMCPG>S(+)DMCPG>(±)DMCPG.
Full table
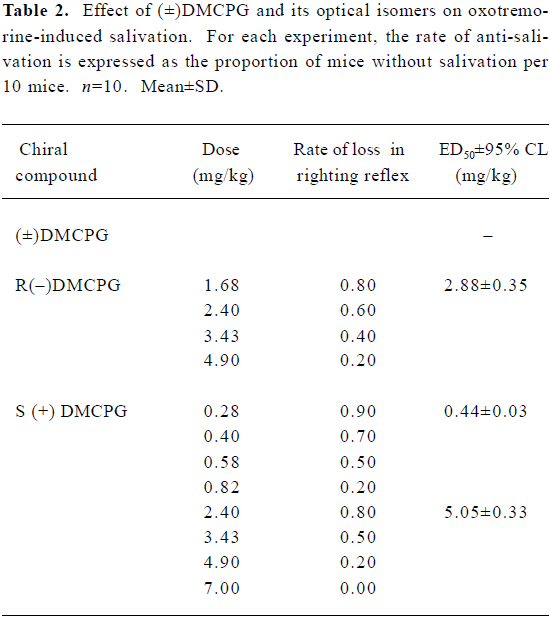
Inhibition of oxotremorine-induced salivation Oxotremorine (sc, 3 mg/kg) induced obvious salivation in mice (n=50), whereas (±)DMCPG and its optical isomers produced anti-salivation effects in a dose-dependent manner when mice were pretreated with these compounds. The ED50±95% CL values for (±)DMCPG and the R(–), S (+) configurations were 2.88±0.35, 0.44±0.03, and 5.05±0.33 mg/kg, respectively, which indicates that the order of potency for inhibiting glandular secretion is R(–)DMCPG>(±)DMCPG>S(+)DMCPG (Table 2).
Full table
Discussion
(±)DMCPG is a derivative of its parent compound CPG, and there is one chiral carbonic atom in the molecular structure of (±)DMCPG, causing the R(–) and S(+) enantiomers. In this study, we compared the pharmacological activities of these enantiomers, to further investigate the relationships between anti-muscarinic activity and muscarinic receptors. Interestingly, for muscarinic acetylcholinic receptors, (±)DMCPG and its optical isomers did not have the same potency trends in the tests.
In the present investigation, the pharmacological activities of (±)DMCPG and its isomers were subjected to comparative radiobinding and functional assays. In the competitive binding assay, R(–)DMCPG was 4- and 2-fold more potent than its racemate and the S(+) configuration in inhibiting the binding of [3H]QNB. These results demonstrate that there was receptor stereo selective action between the muscarinic receptor and R (–)DMCPG. In this case of receptor binding, R(–)DMCPG is the eutomer. The R(–) and S(+) configurations showed positive cooperation (nH>1) but (±)DMCPG had a negatively cooperative (nH=0.42) relationship with the muscarinic receptor. Low Hill numbers are most often attributed to recognition by the antagonist of more than one receptor site or receptor conformation, or to an interaction of the antagonist with a second binding site on the receptor molecule causing a negative cooperative effect for the first site[7–9]. There is a second ligand-binding site on muscarinic receptors. A wide array of compounds is capable of modulating the binding of classical ligands to all five muscarinic subtypes[10–12]. Allosteric modulation of muscarinic receptors has been much investigated[13,14]. Proška and Tuèek (1994) proposed that the binding site for modulators such as alcuronium, gallamine, and related compounds is located near the binding site for classical ligands, but more superficially[15]. Our results imply that (±)DMCPG may act at the second binding site with an allosteric mechanism to muscarinic receptors. (±)DMCPG is composed of the R(–) and S(+) configurations, and R(–) and S(+)DMCPG showed marked central inhibitory effects, but (±)DMCPG had only a weak effect, even at a higher dose. Inversely, the ability of (±)DMCPG to inhibit the contraction of guinea pig ileum induced by carbachol was approximately 10 and 100 times more potent than that of R(–) and S(+)DMCPG. (±)DMCPG was the lowest one bound to the muscarinic receptor, and we can also deduce that the enantiomers must interact with each other to affect its binding characteristics and bioactivities. The allosteric mechanism of these chiral drugs needs to be further explored in subsequent experiments. However, our experiments showed that pharmacological differences exist between the optical isomers. The R(–) configuration was more potent at binding receptors and inhibiting glandular secretion, but had moderate effects on the contraction of smooth muscle. The pharmacological differences may be due to the distribution of different subtypes in different tissues. Muscarinic acetylcholine receptors (mAChR) include five subtypes of receptors (M1–M5). The selective action on the salivary gland of R(–)DMCPG may be related to its subtype-selective effects. Individual members of the mAChR are expressed in a complex overlapping fashion in most tissues and cell types[16]. The M1, M2, and M4 subtypes of the mAchR are the predominant receptors in the central nervous system[17]. The M1 and M3 subtypes are the major muscarinic acetylcholine receptors in the salivary glands and M3 is thought to be more abundant[18,19]. Guinea pig ileum smooth muscle is enriched with muscarinic receptors, the majority of which are of the M2 subtype, and the remaining minority are of the M3 subtype[20,21]. In our experiments, we comparatively studied the anti-muscarinic pharmacological profiles of these compounds to see whether there is any correlation between pharmacological activity and muscarinic subtype selectivity. Fulfilling our expectations, of these three chiral drugs, (±)DMCPG had the greatest ability to inhibit smooth muscle contraction as a muscarinic receptor antagonist, whereas R(–)DMCPG had a moderate effect on smooth muscle contraction, but had the greatest anti-salivary effect and enhancement of sedation effects caused by sub-threshold hypnotic doses of sodium pentobarbital. S(+)DMCPG was less potent in all experimental models. Our results imply that there are subtype-selective mechanisms that correspond to the different pharmacological actions of the compounds. Therefore, further studies are necessary to resolve the underlying actions of (±)DMCPG and its enantiomers with respect to muscarinic subtype receptors.
In conclusion, the present work demonstrated that that there are receptor stereo selective actions between muscarinic receptors and the R(–) configuration of DMCPG. R(–) DMCPG acted as a eutomer relative to its S(+) configuration in the racemate. These differences must be related to subtype selectivity and allosteric mechanisms.
References
- Dai JG, Liu CG, Yu LS, Yang AZ, Jia HB, Wang KN. Antimotion sickness effect of phencynonate hydrochloride in man. Chin J Aerospace Med 1997;8:10-4.
- Deng YJ, Zhang YM. Study on the efficacy of phencynonate hydrochloride tablets in prevention of motion sickness. Chin J New Drugs 2001;10:453-4.
- Wang LY, Zheng JQ, Zhong BH, Ruan JX, Liu KL. Pharmacological profiles of anticholinergic agents, phencynonate hydrochloride and its optical isomers. Acta Pharmacol Sin 2005;26:613-40.
- Yamamura HI, Snyder SH. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci USA 1974;71:1725-9.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folinphenol reagent. J Biol Chem 1951;193:265-75.
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099-108.
- Nathanson NM. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci 1987;10:195-236.
- Hulme EC, Berrie CP, Birdsall NJM, Burgen ASV. Two populations of binding sites for muscarinic receptor antagonists in the rat heart. Eur J Pharmacol 1981;73:137-42.
- Stockton J, Birdsall NJM, Burgen ASV, Hulme EC. Modification of the binding properties of muscarinic receptors by gallamine. Mol Pharmacol 1983;23:551-7.
- Ellis J. Allosteric binding sites on muscarinic receptors. Drug Dev Res 1997;40:193-204.
- Christopoulos A, Lanzafame A, Mitchelson F. Allosteric interactions at muscarinic cholinoceptors. Clin Exp Pharmacol Physiol 1998;25:185-94.
- Lee NH, El-Fakahany EE. Allosteric antagonists of the muscarinic acetylcholine receptor. Biochem Pharmacol 1991;42:199-205.
- Holzgrabe U, Mohr K. Allosteric modulators of ligand binding to muscarinic acetylcholine receptors. Drug Res Today 1998;3:214-22.
- Christopoulos A, Lanzafame A, Mitchelson F. Allosteric interactions at muscarinic cholinoceptors. Clin Exp Pharmacol Physiol 1998;25:185-94.
- Proška J, Tuèek S. Mechanisms of steric and cooperative actions of alcuronium on cardiac muscarinic acetylcholine receptors. Mol Pharmacol 1994;45:709-17.
- Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci 2003;24:414-20.
- Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res 2004;145:59-66.
- Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, et al. M (3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol 2004;558:561-75.
- Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol 2004;66:260-7.
- Ehlert FJ, Thomas EA. Functional role of M2 muscarinic receptors in the guinea pig ileum. Life Sci 1995;56:965-71.
- Honda K, Takano Y, Kamiya H. Pharmacological profiles of muscarinic receptors in the longitudinal smooth muscle of guinea pig ileum. Jpn J Pharmacol 1993;62:43-7.