Effects of astragaloside IV on pathogenesis of metabolic syndrome in vitro1
Introduction
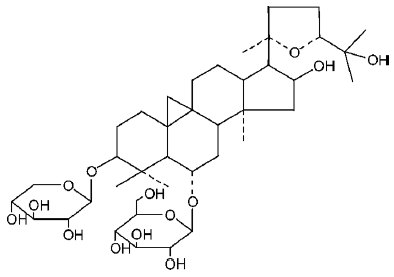
Radix astragali is a herbal remedy widely used in traditional Chinese medicine for the treatment of diabetes, cardiovascular diseases (CVD), and inflammation[1]. Astragalo-side IV (3-0-beta-D-xylopyranosyl-6-0-beta-D-glucopyrano-sylcycloastra-genol; Figure 1) is one of the main active ingredients of radix astragali, which has also been reported to have a range of pharmacological actions, including anti-diabetic[2], anti-hypertensive[3], anti-inflammatory[3], myocardial protective[4], anti-heart failure effects. These pharmacological actions are diverse, so the purpose of the present study was to synthetically study these effects, attempt to link them, and then attempt to find the common underlying mechanisms.
Recent researches have indicated that obesity, insulin resistance, hypertension, dislipidemia, and atherosclerosis always occur together and have common pathogenesis. This cluster of metabolic and CVD risk factors has been termed the “metabolic syndrome”, and was defined by the World Health Organization in 1999[5]. Although the underlying pathogenesis of metabolic syndrome is still not fully clear, a large body of evidence now indicates that insulin resistance may be a central abnormality, and that there is a complicated interplay between insulin resistance, adipocytes and endothelial dysfunction that links the abnormalities of metabolic syndrome[6].
In the present work, we studied the pharmacological action of astragaloside IV from the perspective of metabolic syndrome. We investigated the effects of astragaloside IV on (1) the process of preadipocyte differentiation into adipocytes; (2) the glucose uptake of adipocytes that have become insulin resistant through exposure to high glucose levels; and (3) the peroxisome proliferator-activated receptor-γ (PPARγ) gene expression of preadipocytes. We then investigated the influence of astragaloside IV on endothelial cell (EC) viability loss and apoptosis induced by tumor necrosis factor-α (TNF-α). Additionally, we measured the effect of astragaloside IV on TNF-α-induced intracellular free Ca2+ accumulation in EC.
Materials and methods
Cell culture Preadipocytes were isolated from adipose tissue using a method modified from that of Rodbell[7]. The epididymal adipose tissue from male Sprague-Dawley rats (100–150 g, Zhejiang Center of Laboratory Animals) was removed under sterile conditions and washed in D-Hanks’ solution. Minced tissue was digested with 0.1% type II collagenase (Sigma, St Louis, MO, USA). After incubation at 37 °C for 45 min, the digest was filtered through a 250-μm nylon mesh. The digested tissue was centrifuged at 200×g for 10 min, and mature adipocytes were removed. The pellet was resuspended in D-Hanks’ solution, filtered through a 25-μm nylon mesh, and centrifuged again. The pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal calf serum (FCS; HyClone, Logan, UT, USA). Cells were plated in 60-mm culture dishes at a density of 1.5×105–3×105 cells/mL. The cells were then subcultured every 3 d.
Primary human umbilical vein endothelial cells (HUVEC) were cultured using the method of Jaffe et al[8]. Briefly, human umbilical cords were acquired aseptically from a hospital, and HUVEC were isolated by using 0.1% type II collagenase digestion. Cells (at a density 5×104 cells/mL) were primarily cultured in RPMI-1640 (Gibco) containing 20% FCS in 96- or 24-well culture plates (Nunc, Roskilde, Denmark) previously coated with 0.02% gelatin. Cells grew to confluence in 2–3 d. Both types of cells were maintained in a CO2 incubator with 95% air and 5% CO2 at 37 °C.
Assessment of preadipocyte differentiation Preadipo-cytes were plated in 24-well culture plates at a density of 5×104 cells/well. After 1 d, the cells reached maximal conflu-ence and then were treated individually with different concentrations of astragaloside IV (3, 10, and 30 µg/mL) (Zhejiang Conba Pharmaceutical Co, Hangzhou, China, purity above 98%) for 3 d in 500 µL of DMEM containing insulin 1 µmol/L and 10% FCS. After the removal of insulin and astragaloside, the cells were further cultured for 8 d, during which the medium was renewed every 3 d. After a total of 12 d, adipogenesis was used as an indicator of the effects of astragalo-side on preadipocyte differentiation. Cells cultured in plates were washed 3 times with phosphate-buffered saline (PBS), and fixed with 10% formalin in PBS for 1 h. After being stained with 0.1 mg/mL oil red O solution for 2 h, the cells were washed 3 times with water, and all water was then vaporized (32 °C for 45 min). The precipitation was dissolved by adding 100 µL isopropanol. The absorbance at 510 nm was measured by using a microplate reader (Bio-Rad 550, Hercules, CA, USA)[9]. For the vehicle control, 1% Me2SO was used (normal group), and for the positive control, rosiglitazone 3 µg/mL (GlaxoSmithKline, Philadelphia, PA, USA) was used.
Analysis of mRNA expression by reverse transcription–polymerase chain reaction Preadipocytes were plated on 6-well culture plates, as described in the previous paragraph. Total RNA was isolated from adipocytes at d 7 by using a Trizol total RNA extraction kit (Shanghai Sangon, Shanghai, China). The reverse transcription-polymerase chain reaction (RT-PCR) was carried out as described previously[10]. The PCR primer sequences were as follows: aP2 forward 5'-GACCTGGAAACTCGTCTCCA-3' and reverse 5'-CATGA-CACATTCCACCACCA-3'; PPARγ forward 5'-AACCGGAA-CAAATGCCAGTA-3' and reverse 5'-TGGCAGCAGTGGAA-GAATCG-3'. Total RNA was reverse transcribed to cDNA by using reverse transcriptase (Promega, Madison, WI, USA) at 37 °C for 1 h . The cDNA was then amplified by using Taq polymerase (Takara, Shiga, Japan) with each primer. The temperature program for the amplification was as follows: 30 s at 94 °C, 30 s at 54 °C and 1 min at 72 °C for 23 cycles (aP2) or 35 cycles (PPARγ). The RT-PCR products were electrophoresed on 1.4% agarose gels, stained with ethidium bromide, and revealed by using ultraviolet irradiation. The RT-PCR products were semi-quantitated by using Gel Doc 2000 (Bio-Rad) and Quantity One software (Bio-Rad). The concentration of PPARγ mRNA was expressed as the ratio of the mRNA expression of PPARγ to that of β-actin.
Glucose uptake study Glucose uptake by the adipocytes was determined by measuring the transport of 2-deoxyglu-cose (2-DG) into the cells, as described previously[11], with some minor modifications. Preadipocytes were induced to differentiate into adipocytes by incubation in a medium containing 10% FCS, 1 µmol/L insulin, 1 µmol/L dexamethasone, and 0.5 mmol/L isobutyl-methylxanthine (IBMX; Sigma) for 2 d. Then the medium was switched to one containing 10% FCS and 1 µmol/L insulin for 2 d, and then again to a normal 10% FCS medium for 2 d. After 6 d, almost all of the preadipocytes had differentiated into adipocytes. These cells were plated on 24-well plates, and incubated with DMEM medium containing a high concentration of glucose (35 mmol/L) (to induce insulin resistance) and varying concentrations of astragaloside IV (3, 10, and 30 μg/mL), rosiglitazone (3 µg/mL) or vehicle for 48 h. Then adipocytes were serum-deprived for 4 h, and incubated in N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES; Hyclone, Logan, UT, USA) with 0.1 µmol/L insulin, 1 µCi/mL [2-3H] deoxyglucose (Beijing Atom HighTech Co, Beijing, China) and 125 µmol/L unlabeled 2-DG (Sigma) for 1 h. The cells were then extensively washed with cold HEPES. After 100 µL NaOH solution (0.2 mol/L) was added, the solution was neutralized by the addition of 100 µL HCl (0.2 mol/L). An aliquot of 150 µL of solution was aspirated, and the radioactivity therein was measured by liquid scintillation counting (Wallac 1414, Turku, Finland).
Assessment of endothelial cell apoptosis The effect of astragaloside IV on TNF-α-induced apoptosis of EC[12] was investigated by using an annexin-V kit (Caltag, Burlingame, CA, USA) and flow cytometer (FACsort, Becton-Dickinson, San Jose, CA, USA). EC were plated in 24-well plates at a density of 1×105 cells/well. After 24 h the cells were treated individually with different concentrations of astragaloside IV (3, 10, and 30 µg/mL), rosiglitazone (30 ng/mL) or vehicle for 48 h in 200 µL of RPMI-1640 medium containing 1 µmol/L insulin, 10% FCS and 40 ng/mL TNF-α (Sigma). Following culture, cells were harvested and washed twice with D-Hanks’ solution, then cells were collected by centrifugation at 240×g and resuspended in 1×binding buffer at a concentration of 1×106 cells/mL. Cells in binding buffer (100 µL) were transferred to a 5-mL culture tube, and stained with 5 µL fluorescein isothiocyanate (FITC)-conjugated annexin V and 10 µL propidium iodide (PI; 50 µg/mL). After 15-min incubation at 20–25 °C in the dark, 200 µL of 1×binding buffer was added to the cells in each tube, and the mixture was analyzed on a flow cytometer. Forward scatter (FSC) and side scatter (SSC) were collected in linear mode and FL1 and FL2 in log mode. At least 10 000 cells were collected for each sample, and the data were analyzed using CellQuest software (Becton-Dickinson). This assay identified normal cells as PI-negative and annexin V (FITC)-negative, and apoptotic cells as PI-negative and annexin V (FITC)-positive.
Assessment of endothelial cell viability The effect of astragaloside IV on the TNF-α-induced viability loss of EC was assessed by using the water-soluble tetrazolium-1 (WST-1, Dojindo Laboratories, Kumamoto, Japan) colorimetric assay[13]. EC were plated on 96-well plates at a density of 1×104 cells/well. After 12 h, the cells were treated individually with different concentrations of astragaloside IV (3, 10, and 30 μg/mL), rosiglitazone (30 ng/mL) or vehicle for 48 h in 100 μL of RPMI-1640 medium containing 40 ng/mL TNF-α (Sigma), 1 µmol/L insulin and 10% FCS. After the drug treatment, WST-1 reagent was added to each well and the cells were incubated for 2 h at 37 °C. Following incubation, absorbance at 450 nm was determined by using a microplate reader.
Fluo-3 fluorescence measurements Fluo-3 fluorescence was measured using a method described elsewhere[14] with minor modifications. Briefly, cells were washed 3 times with RPMI-1640 without FCS. Intracellular free Ca2+ was labeled with Fluo-3 AM (Molecular Probes, Eugene, OR, USA). Cells were incubated individually with different concentrations of astragaloside IV (3, 10, and 30 µg/mL) or vehicle in 50 μL RPMI-1640 medium containing 5 µmol/L Fluo-3 AM at 37 °C for 30 min. The fluorescence intensity was measured by using laser confocal scanning microscopy (Zeiss LSM510, Jena, Germany). The Fluo-3 AM was excited at 488 nm by laser and emissions between 515-560 nm were obtained. Images of 512×512 pixels in size were acquired with a 20× objective. The acquisition rate was 1 frame (512×512 pixels) per 5 s. After the parameters had been adjusted appropriately, laser scanning was used to obtain a time series of images. The TNF-α (40 ng/mL) was added after 3 s. The images obtained were quantitatively analyzed for changes in fluorescence intensity within regions of interests (ROI) using the Zeiss LSM software. By selecting ROI, information about these areas could be obtained and information such as intensity and histograms could be further extracted. Increases in intracellular free Ca2+ were expressed as the ratio of fluorescence intensity of Fluo-3 AM to baseline fluorescence intensity (F/F0).
Statistical analysis Data are presented as mean±SD. Statistical comparisons between groups were carried out using Student’s t-test. P<0.05 was considered significant.
Results
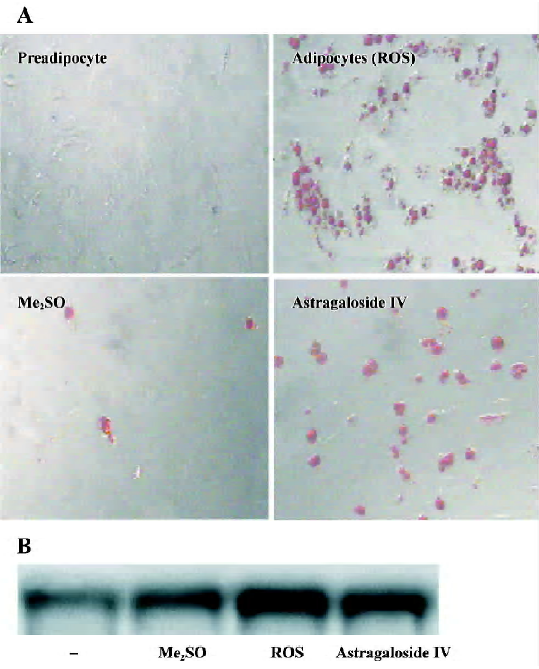
Differentiation of preadipocytes Rosiglitazone was used as the positive control, because it belongs to a novel class of anti-diabetic agents, the thiazolidinediones (TZD), which have therapeutic potential to improve metabolic syndrome in addition to diabetes[15]. The insulin-induced increases in lipids in preadipocytes in the groups treated with astragalo-side IV 3, 10, and 30 µg/mL or rosiglitazone 3 µg/mL were significantly greater than those observed in the normal group. There was no significant difference among the groups treated with different concentrations of astragaloside IV (Table 1). The adipogenesis-modulating activity of astragalo-side IV was confirmed by using a photograph (oil red O staining) and RT-PCR analysis of adipocyte-specific aP2 gene expression (Figure 2A, 2B). This observation suggests that astragaloside IV can potentiate insulin-induced preadipocyte differentiation.
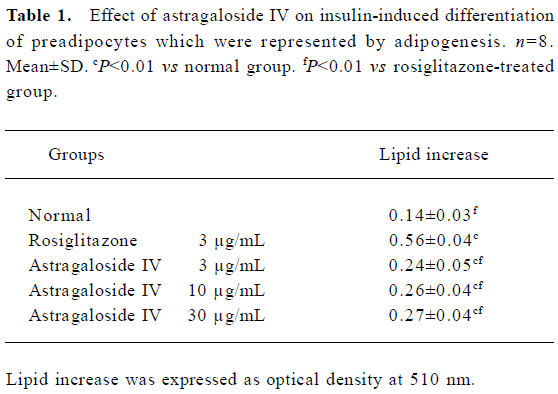
Full table
Insulin-induced glucose uptake of adipocytes Adipo-cytes incubated in medium containing a high concentration of glucose became insulin resistant. The glucose uptake of control group cells descended by 44%, compared to the normal group (Table 2). The insulin-induced glucose uptake of the group treated with astragaloside IV 30 µg/mL was significantly higher than that of the control group and significantly lower than that of the group treated with rosiglitazone (3 µg/mL) and the normal group. It indicates that astragaloside IV can potentiate the uptake of glucose by adipocytes that have become insulin resistant by exposure to high concentrations of glucose.
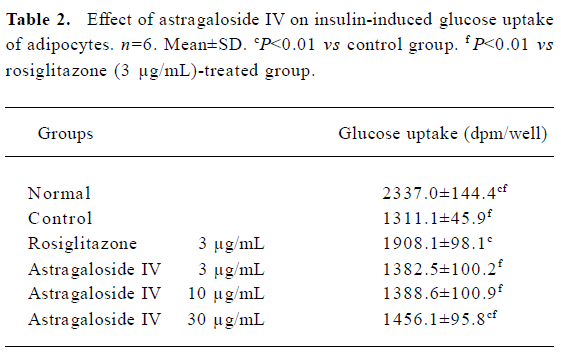
Full table
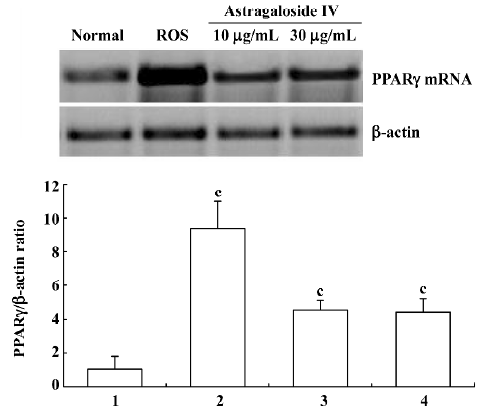
PPARγ expression in preadipocyte At d 7, the concentration of PPARγ mRNA transcripts in preadipocytes of the group treated with astragaloside IV 10 µg/mL or 30 µg/mL was significantly higher than that in the normal group. There were no significant differences between the groups treated with astragaloside IV 30 µg/mL or 10 µg/mL (Figure 3). This finding shows that astragaloside IV promotes PPARγ mRNA expression.
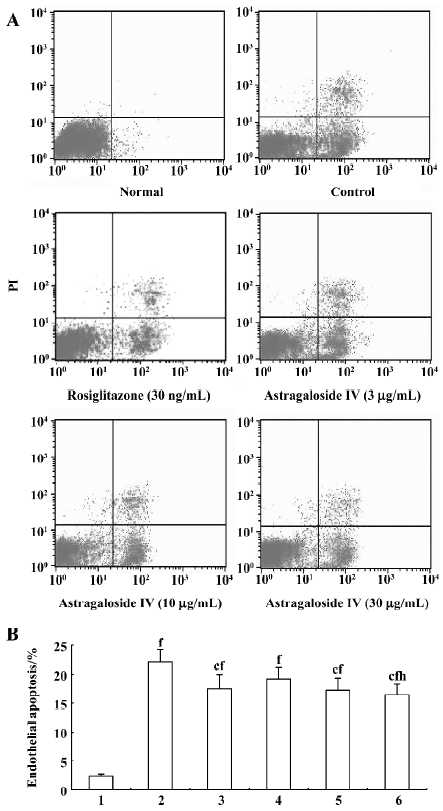
Endothelial cells dysfunction Apoptosis of endothelial cells was significantly increased by 19.7% in the group treated with TNF-α (40 ng/mL) relative to the normal group. The percentage of apoptotic endothelial cells in the groups treated with 10 and 30 µg/mL astragaloside IV and rosiglitazone (30 ng/mL) was significantly lower than that in the control group and significantly higher than that in the normal group. There was no significant difference between the groups treated with 30 µg/mL and 10 µg/mL astragaloside IV, but the percentage of apoptotic endothelial cells in the group treated with astragaloside IV 30 μg/mL was significantly lower than that in the group treated with 3 µg/mL astragaloside IV (Figure 4A, 4B).
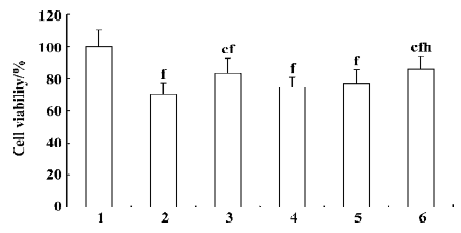
Endothelial cell viability decreased by 30% relative to the normal group after exposure to TNF-α (40 ng/mL) for 48 h. There was no significant difference with respect to cell viability between the groups treated with rosiglitazone (30 ng/mL) and 30 µg/mL astragaloside IV, but cell viability in these groups was significantly higher than that in the control group, and significantly lower than that in the normal group. The groups treated with 3 µg/mL and 10 µg/mL astragaloside IV did not have significantly higher cell viability than the control group. These data indicate that astragaloside IV dose-dependently prevents endothelial cells apoptosis and viability loss due to TNF-α (Figure 5).
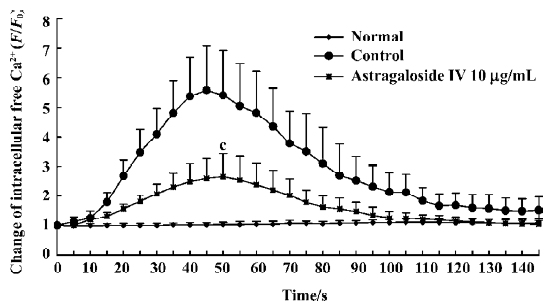
Ca2+ elevation induced by TNF-α in EC After being stimulated with TNF-α, the fluorescence value of Ca2+ in EC rapidly increased and arrived at a peak value (5.6-fold) within 60 s, then slowly decreased near to the basal level (Figure 6). When cells were pretreated with astragaloside IV, the peak fluorescence intensity corresponding to TNF-α-induced Ca2+ elevation was significantly reduced by 2.9-fold relative to cells treated with TNF-α alone. It suggests that astragaloside IV has antagonistic effects on TNF-α-induced Ca2+ elevation in EC.
Discussion
The results of the present study showed that astragalo-side IV could potentiate the insulin-induced differentiation of preadipocytes. Recently, adipocytes have been recognized to play an active role in glucose and lipid metabolism by depositing fat and secreting polypeptides such as leptin, resistin, and adiponectin[16]. Preadipocytes differentiate into adipocytes, and newly differentiated lean adipocytes are more sensitive to insulin and secrete fewer harmful mediators (free fatty acids, TNF-α, interleukin-6, etc) than do old obese adipocytes[17]. Therefore preadipocyte differentiation may play an important role in lipid and glucose metabolism. This effect of astragaloside IV is consistent with that of rosigli-tazone, which can also potentiate preadipocyte differentia-tion.
Many researches indicate that insulin resistance may be the central abnormality of metabolic syndrome[5,6]. The presence of obese adipocyte-derived mediators (free fatty acids, resistin, TNF-α, etc) can lead to the development of insulin resistance. Then, through direct and/or indirect mechanisms, insulin resistance causes hyperglycemia, endothelial dys-function, hypertension and arteriosclerosis[18]. In the present study, we found that similar to rosiglitazone, astragaloside IV, at a high concentration, could potentiate the glucose-uptake of adipocytes in which insulin resistance had been induced by exposure to high concentrations of glucose. These results are compatible with previously research in which it was found that astragalus or astragalus polysaccharides influenced carbohydrate metabolism and preadipo-cyte differentiation[19,20].
To understand the mechanism by which astragaloside IV potentiates preadipocyte differentiation and reduces insulin resistance, the effect of astragaloside IV on PPARγ gene expression was investigated. PPARγ is a nuclear receptor that plays a regulatory role in the expression of genes related to preadipocyte differentiation, insulin sensitivity, and inflammation[21]. Rosiglitazone is thought to mainly exert its effect through the activation of PPARγ. In the present study, we found that astragaloside IV significantly promoted PPARγ mRNA expression. Thus, astragaloside IV may, through stimulating PPARγ expression, potentiate preadipocyte differentiation and improve insulin sensitivity. Further investigations are needed to confirm this finding.
EC are known to play a pivotal role in vascular function and remodeling. Endothelial dysfunction may link lipid and glucose metabolic disorders and CVD[22]. Several mechanisms may contribute to endothelial dysfunction in metabolic syndrome, including insulin resistance, and the action of inflammatory cytokines. There is reasonable evidence showing that metabolic syndrome is an inflammatory state, which is associated with an increase in plasma inflammatory cytokine concentrations, particularly that of TNF-α[23]. The inflammatory cytokines may be primarily over-released from the obese adipocytes[24]. Accordingly, we used TNF-α to induce inflammatory endothelial dysfunction and investigated the effects of astragaloside IV on apoptosis and viability loss in EC.
Apoptosis is a process for disposing of senescent, injured, or redundant cells through self-destruction, and has been demonstrated to play a role in EC loss during hypertension[25]. TNF-α-induced apoptosis of EC also plays an essential role in the pathological processes of atherosclerosis[26]. Therefore, apoptosis represents an important process in the pathogenesis of endothelial dysfunction. In the present study, apoptotic cells were assessed using annexin V labeling. We showed that astragaloside IV could reduce the TNF-α-induced apoptosis of EC. Additionally, by using the WST-1 assay to measure EC viability, we showed that astragaloside IV could significantly prevent TNF-α-induced viability loss in EC. These results are compatible with previous findings showing that astragaloside IV can inhibit histamine-induced inflammation in EC[27].
Ca2+ is a major second messenger, and intracellular free Ca2+ overload can lead to dysfunction in EC, which has been implicated in the signaling pathways inducing apoptosis[28]. Also, research has shown that treatment of EC with a Ca2+ chelator (BAPTA-AM) partially prevents TNF-α-induced apoptosis[29]. Data from the present study showed that astragaloside IV inhibited intracellular free Ca2+ accumulation in EC subjected to TNF-α. These findings are compatible with the recent finding that astragaloside IV can reduce the excessive accumulation of intracellular calcium within myocardial cells[4]. This phenomenon may partially explain the anti-apoptotic effect of astragaloside IV.
In conclusion, the results of the present study indicate that astragaloside IV can potentiate preadipocyte differen-tiation, improve insulin resistance in adipocytes exposed to high concentrations of glucose, and prevent endothelial apoptosis and viability loss. These effects are probably partly due to the promotion of PPARγ expression and partly due to inhibition of abnormal EC intracellular free Ca2+ accumulation. Thus, the present study provides new insights into the mechanism by which astragaloside IV exerts its effects.
References
- Wu F, Chen X. A review of pharmacological study on Astragalus membranaceus (Fisch) Bge. Zhong Yao Cai 2004;27:232-4.
- Jiang QL, Jiang JW, Lu XX. Effect of astragaloside IV on glucagon-like peptide-1. Chin J Gerontol 2003;23:52-3.
- Zhang YD, Wang YL, Sheng JP. Effect of astragaloside IV on inflammatory and hypertension. Acta Pharmacol Sin 1984;19:333-7.
- Cao Q, Li ZP. Effects of astragaloside IV on myocardial calcium transport and cardiac function in ischemic rats. Acta Pharmacol Sin 2002;23:898-904.
- Bo I. A major health hazard: the metabolic syndrome. Life Sci 2003;73:2395-411.
- Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res 2003;11:1278-89.
- Rodbell M. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964;239:375-80.
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 1973;52:2745-56.
- Ramirez ZJL, Castro MF, Kuri HW. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992;97:493-7.
- Iijima K, Yoshizumi M, Ako J, Eto M, Kim S, Hashimoto M, et al. Expression of peroxisome proliferator-activated receptor γ (PPARγ) in rat aortic smooth muscle cells. Biochem Biophys Res Commun 1998;247:353-6.
- Olefsky JM. Mechanisms of the ability of insulin to activate the glucose-transport system in rat adipocytes. Biochem J 1978;172:137-45.
- Spyridopoulos I, Brogi E, Kearney M, Sullivan AB, Cetrulo C, Isner JM, et al. Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-α: balance between growth and death signals. J Mol Cell Cardiol 1997;29:1321-30.
- Fukunaga Y, Itoh H, Doi K, Tanaka T, Yamashita J, Chun TH, et al. Thiazolidinediones, peroxisome proliferator-activated receptor γ agonists, regulate endothelial cell growth and secretion of vasoactive peptides Atherosclerosis 2001;158:113-9.
- Wang LZ, Zhang QZ, Hu XZ, Lun N, Zhu FH. Verapamil, cyproheptadine, and anisodamine antagonized (Ca2+)i elevation induced by TNF-α in a single endothelial cell. Acta Pharmacol Sin 2001;22:918-22.
- Fujiwara T, Horikoshi H. Troglitazone and related compounds: therapeutic potential beyond diabetes. Life Sci 2000;67:2405-16.
- Peter J. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 2004;53 Suppl 1:S143-51.
- Okuno A, Tamemoto H, Tobe K. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 1998;101:1354-61.
- Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes 2004;53:2735-40.
- Wang SH, Wang WJ, Wang XF, Chen W. Effect of Astragalus polysaccharides and berberine on carbohydrate metabolism and cell differentiation in 3T3-L1 adipocytes. Chin J Int Tradit West 2004;24:926-8.
- Yang LH, Qu DJ, Feng ZK, Lu J, Liu Y, Wu WJ. Effect of Radix astragali on insulin sensitivity in high-fat-fed rats. Acad J Sec Milit Med Univ 2004;25:407-9.
- Kota BP, Huang TH, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Res 2005;51:85-94.
- Torp-Pedersen C, Rask-Madsen C, Gustafsson I, Gustafsson F, Kober L. Diabetes mellitus and cardiovascular risk: just another risk factor? Eur Heart J 2003; Suppl 5–6: F26–32.
- Winkler G, Lakatos P, Salamon F, Nagy Z, Speer G, Kovacs M, et al. Elevated serum TNF-alpha level as a link between endothelial dysfunction and insulin resistance in normotensive obese patients. Diabet Med 1999;16:207-11.
- Rajala MW, Scherer PE. Scherer minireview. The adipocyte: at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 2003;144:3765-73.
- Gobe G, Browning J, Howard T, Hogg N, Winterford C, Cross R. Apoptosis occurs in endothelial cells during hypertension-induced microvascular rarefaction. J Struct Biol 1997;118:63-72.
- Kaiser S, Hennig B, Toborek M. Apoptosis of vascular endothelial cells in response to TNF and lipid treatments. Atherosclerosis 1997;134:243-4.
- Fan Y, Wu DZ, Hu ZB. Effects of astragaloside IV on the enhance of endothelial permeability induced by histamine. Chin J Bas Med TCM 2001;7:36-8.
- Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol 2001;33:1673-90.
- Toborek M, Blanc EM, Kaiser S, Mattson MP, Hennig B. Linoleic acid potentiates TNF-mediated oxidative stress, disruption of calcium homeostasis, and apoptosis of cultured vascular endothelial cells. J Lipid Res 1997;38:2155-67.