Relationship between atrial natriuretic peptide-immunoreactive cells and microvessels in rat gastric mucosa1
Introduction
Atrial natriuretic peptide (ANP) was isolated from the atrium by de Bold et al in 1981[1], and since then, brain natriuretic peptide, C-type natriuretic peptide, dendroaspis natriuretic peptide, micrurus natriuretic peptide, and ventricular natriuretic peptide have also been found. These peptides are actually distributed all over the body, not only in the heart[2–5]. ANP regulates a variety of physiological functions, including natriuresis, diuresis and vasodilation. Although ANP is synthesized primarily in the heart as a cardiac hormone, the fact that ANP and its receptor are also expressed in numerous extracardiac tissues (eg lung, thymus, and gastrointestinal tract tissues) suggests that ANP may play an important role as a regional autocrine and/or paracrine regulatory peptide in extracardiac tissues[6]. By using immunohistochemistry and in situ hybridization, it has been found that ANP gene expression is localized in the rat gastric antrum in enterochromaffin cells (EC cells) in the lower portion of the antropyloric glands[7–9].
Our previous studies indicated that the natriuretic peptide receptor (NPR) existed in different regions of the gastric mucosa, that its density was greatest in rat gastric antrum, and that natriuretic peptide significantly inhibited the spontaneous contraction of gastric smooth muscles in rats, guinea-pigs and humans[10,11]. EC cells may transport their synthesized product through their long cytoplasmic processes towards target cells; for example, ANP is transported from EC cells to the smooth muscle layer by microvessels . It has been suggested that blood capillaries are the targets for the long cytoplasmic processes of EC cells[12]. However, the distribution of ANP-synthesizing cells and the relationship between the distribution of ANP-synthesizing cells and microvessel density in different regions of the stomach is not clear. Therefore, in the present study, the morphological distribution and ultrastructural localization of ANP-synthesizing cells were studied by using postembedding immuno-electron microscopy techniques, and the relationship between the distribution of ANP-synthesizing cells and microvessel density was investigated by using histochemistry techniques in the rat stomach.
Materials and methods
Animals Wistar rats (obtained from the Experimental Animal Center of Yanbian University College of Medicine) of both sexes weighing 240–260 g (5 months old; normal diet; n=18) were anaesthetized with lethal doses of pentobarbital sodium (30 mg/kg) injected into the abdominal cavity. The abdomen of each rat was opened along the midline, and the stomach was opened along the major curvature. Because rat stomachs have significant regional differences in structure, we separated the stomach into 3 regions in our analyses: the fundus or proximal region, the body or midregion, and the antrum or distal region.
Immunohistochemistry Freshly excised pieces of atrium and stomach (n=18) were fixed in 10% formaldehyde fixative and embedded in paraffin. Sections (5 µm) were deparaffinated, rehydrated and incubated with 0.3% hydrogen peroxide in methanol for 15 min at room temperature to block endogenous peroxidase activity. After two washes with phosphate-buffered saline (PBS) for 5 min, the tissue sections were incubated at 37 oC for 20 min with blocking solution. The sections were then incubated at 37 oC for 2 h with the primary antibody, rabbit antirat ANP (sc-20158, Santa Cruz Biotechnology, California; 1:100 dilution). After 2 washes with PBS (0.01 mol/L; pH 7.4) for 10 min, the tissue sections were incubated at 37 oC for 30 min with biotin-antirabbit IgG. The sections were washed twice with PBS for 5 min and incubated in streptavidin-horseradish peroxidase for 30 min. Then the sections were washed twice with PBS for 5 min and incubated in a metal-enhanced 3,3-diamino-benzidene solution for 15 min, then washed twice with distilled water and counterstained with hematoxylin. Negative control sections were incubated with normal rabbit serum instead of the primary antibody. Cells that stained positive for ANP-synthesis contained red-brown granules, which, using microscopy, were found to be mainly located in the cell cytoplasm. At least 5 high-power (×400) fields were chosen randomly and cells were counted in the lower part of the mucosa, where the ANP-synthesizing cells were densely distributed. The distribution ratio for ANP-synthesizing cells was calculated by dividing the number of positive cells by the total number of cells, then expressing this value as a percentage. Counting and analysis was carried out using the CMIAS image analysis system (Bei Hang, Beijing, China), and the mucosa was photographed using an Olympus (Nagano, Japan) PM-10AD photomicrographic system.
Immunoelectron microscopy Freshly excised stomach mucosal and atrial myocytes (1 mm3 blocks) were fixed in 10% paraformaldehyde fixative, then dehydrated and embedded in Epon-812 resin (Marivac Ltd, Halifax, Nova Scotia). Tissue sections (70 nm) were incubated with 0.1% hydrogen peroxide solution in methanol for 30 min at room temperature. After 2 washes with distilled water for 10 min the tissue sections were incubated at room temperature for 1 h with blocking solution, and then tissue sections were incubated again at 4 oC for 36 h with the primary antibody, rabbit antirat ANP (sc-20158, Santa Cruz Biotechnology; 1:90). Negative control sections were incubated with normal rabbit serum instead of primary antibody, then after 2 washes with PBS for 5 min, the tissue sections were incubated at room temperature for 1 h with protein labeled by A-10 nm colloidal gold (Sigma, Louis, Missouri). The sections were washed twice with distilled water for 5 min, then stained with urenyl acetate and lead citrate for 5 min, respectively, and observed under an 80 kV electron microscope (JEM-1200EX, JEOL, Tokyo, Japan).
Histochemistry Freshly excised rat stomach tissues were fixed with paraformaldehyde fixative (formaldehyde: potassium, 1:4) at 4 oC for 36 h. The tissues were then fixed in 3% potassium bichromate fixative for 12 h at room temperature and embedded in paraffin. Histochemical staining for ANP-synthesizing cells was performed by using the chromaffin staining method. After 2 washes with distilled water for 20 min, the tissues were stained at room temperature for 20 min with 1% toluidine solution, staining for enterochromaffin cell. The sections were washed twice with distilled water for 10 min and stained at room temperature for 15 min with 1% saffron solution. The positive cells contained brown granules, which, on microscopy, were found to be mainly located in the cell cytoplasm. At least 5 high-power (×400 field) fields were chosen randomly and the cells in the fields were observed and counted. The density of positive cells was calculated by using a light microscope (Olympus BH-2) and the CMIAS image analysis system.
Tannic acid-ferric chloride stain To investigate the density and distribution of gastric microvessels in different regions of stomach, the TA-Fe staining method was used. Wistar rats were first perfused with 2% compound fixative tannic acid solution, the stomachs were taken out and frozen, then cut into sections. The sections were immersed in 2% ferric chloride solution at room temperature for 20 min for revealing the microvessels. The blood vessels were clearly visualized by using the TA-Fe staining method, and could be observed under a light microscope. The density and distribution of the vessels were measured and analyzed by using the CMIAS image analysis system, and photographed using an Olympus PM-10AD photomicrographic system according to the method of Kong et al[13].
Data analysis Data are expressed as mean±SD, and the two-tailed χ2 test was used to examine the correlation between ANP-synthesizing cells and microvessel density. Statistical significance was estimated by using the t-test. Differences were considered significant when the P-value was less than 0.05. All the calculations were performed by using SPSS 11.0.
Results
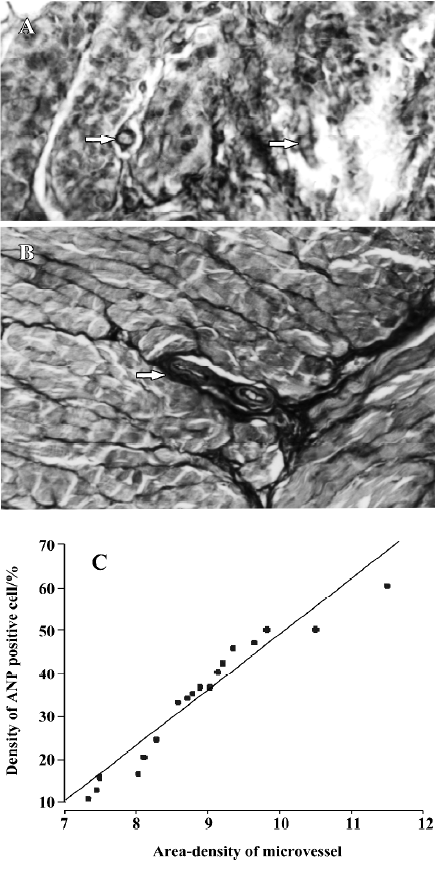
Expression of ANP-synthesizing cells in the rat stomach In the positive control, ANP-synthesizing cells were present at high density in the cytoplasm of the atrial myocytes (as red-brown granules; Figure 1A). ANP-synthesizing cells were also expressed in the gastric mucosa, and the positively-stained granules were localized in the cytoplasm in cells in the basal portion of the fundus glands (Figure 1B, 2A). In the negative control, for which normal rabbit serum was substituted for primary antiserum, no positive staining for ANP-synthesizing cells was observed (Figure 2B). The shape of the individual ANP-synthesizing cells was variable: round, pyramidal and flask shapes were all found. The general epithelial appearance of these cells was typically endocrine, and most immunoreactivity was localized in the basal portion of the stomach. No ANP-synthesizing cell was detected in the lamina propria, submucosa or smooth muscle.
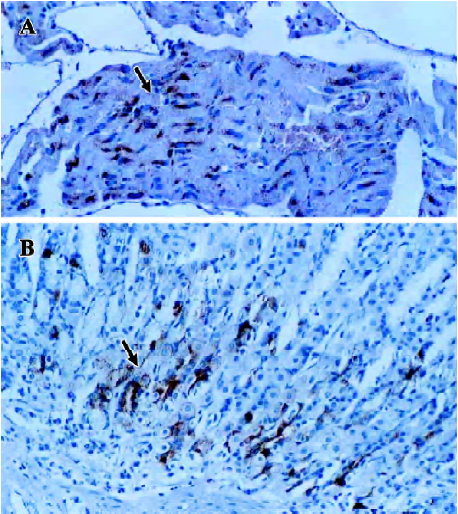
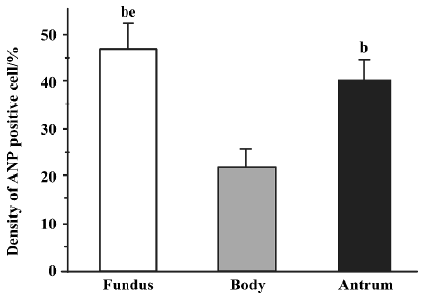
Distribution of ANP-synthesizing cells in rat gastric mucosa The distribution of ANP-synthesizing cells (EC cells) in the different regions of the gastric mucosa was investigated by using histochemical techniques. Consecutive serial sections of rat gastric mucosa were stained for chromaffin, and the chromaffin granules (brown granules) were found to be localized in the EC cell cytoplasm. No chromaffin granules were detected in the lamina propria, submucosa, or smooth muscle, or in the negative control. The distribution of EC cells in gastric mucosa was further examined by staining for chromaffin. Three histologically distinct regions (fundus, body and antrum) were found in the distribution of EC cells in rat gastric mucosa. EC cells are found in the mucosa layer, and their density was greatest in the fundus (46.7%±5.3%; Mean±SD), intermediate in the antrum (40.1%±4.5%), and lowest in the body (21.6%±3.6%) (Figure 3; n=18).
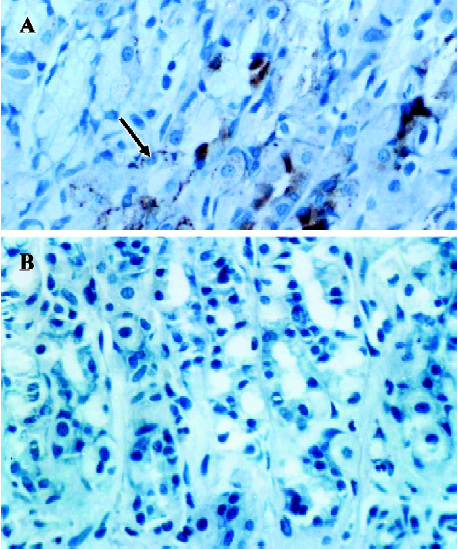
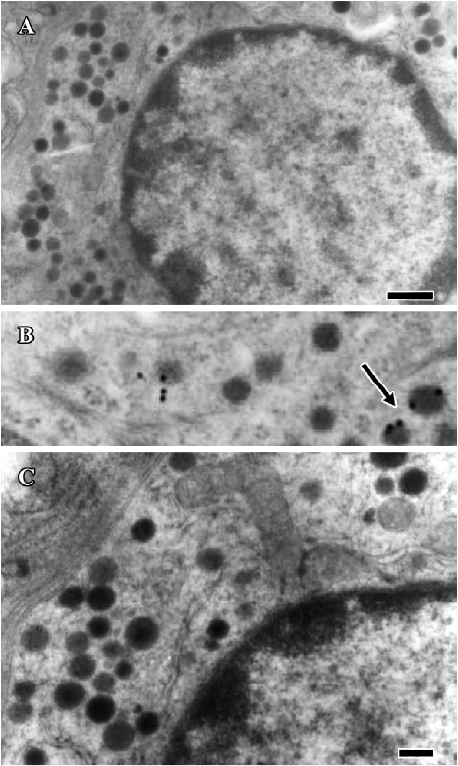
Identification of ANP-synthesizing cells in the rat stomach Immunogold labeling was localized in the endocrine granules of EC cells, which belong to the disperse or diffuse neuroendocrine system (DNES) in the gastric glands (Figure 4B). These results indicate that EC cells synthesize and secrete ANP in rat gastric mucosa. In the negative control, in which normal rabbit serum was substituted for anti-ANP antiserum, no positive staining was observed (Figure 4A, 4C).
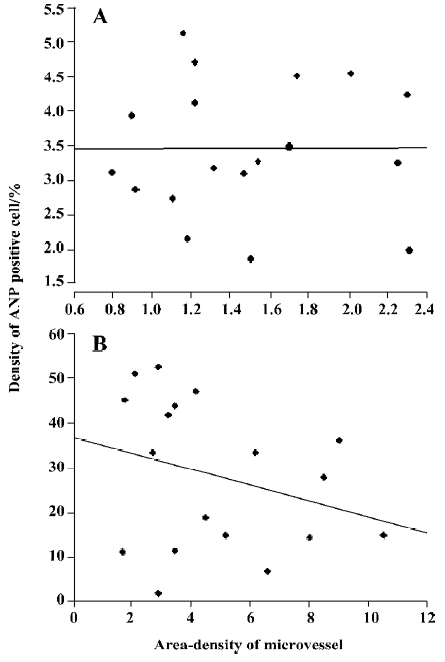
Relationship between ANP-synthesizing cells and microvessel density The gastric mucosa was cut into cross-sections, and microvessels were stained successfully by using the TA-Fe method[12,13]. The microvessels were exhibited in winding state, and they could be clearly observed in 3 dimensions (Figure 5A). Some microvessels were found scattered in the antral mucosa, and some branch arteries from the large vessels ran into the basal glands of the gastric mucosa (Figure 5B). The density of microvessels varied depending on location; in the areas in which more basal glands existed, more microvessels were distributed in the rat gastric mucosa. In order to investigate the relationship between the distribution of ANP-synthesizng cells and microvessel density in the gastric mucosa, data were analyzed by using SPSS 11.0. There was a significant positive relationship between the percentage of ANP-synthesizing cells and microvessel density in the antral mucosa of rats (r=0.53, P<0.05, Figure 5C), but there was a negative relationship in the body mucosa (Figure 6A; n=18, r =–0.2914, P>0.05) and fundus mucosa (Figure 6B; n=18, r=0.3880, P>0.05).
Discussion
In our previous study, the distribution of natriuretic peptide receptors (NPR-A and B) in different regions of rat stomach was investigated by using radioautograph techniques[9,10]. Furthermore, we found that natriuretic peptide inhibited gastric smooth muscle contractions in humans, rats and guineapigs[9,10]. NPR exists in both the mucosal and muscle layers, and the density of NPR in the muscle layer is greatest in the antrum, intermediate in the body, and lowest in the fundus. In the present study, ANP-synthesizing cells were localized, and we verified by using immunohistochemistry and postembedding immunoelectron microscopy techniques that that EC cells synthesized ANP in rat gastric mucosa. ANP-synthesizing cells exist in different regions of the stomach, and their density in the mucosal layer was the greatest in the gastric fundus, intermediate in the antrum, and lowest in the body. There was a positive relationship between the percentage of ANP-synthesizing cells and the density of microvessels in the antral mucosa, but there was a negative relationship in the fundus and body mucosa. Our study suggests that ANP synthesized by EC cells may play an important role in the inhibitory regulation of gastrointestinal motility, perhaps not only as a regional autocrine and/or paracrine regulator, but also as an endocrine regulatory peptide.
ANP-expressing myoendocrine cells are most densely distributed in the right atrium, are found to a lesser extent in the left atrium, and are almost absent in the left ventricle[14,15]. Gower et al[16] and Vuolteenaho et al[17] demonstrated that the gene for ANP was expressed in specific regions of the rat gastrointestinal tract. It has been found immunohisto-chemically that EC cells in the antral and small and large intestinal mucosa, besides being serotonin positive, are also ANP positive[7,8]. However, it was not clear which cells in the gastric body and fundus mucosa manufactured ANP. In the present study we demonstrated immunohistochemically that all EC cells in the gastric fundus, antrum and body mucosa were ANP positive. EC cells are an abundant type of enteroendo-crine cell that contain serotonin and occur throughout the gastrointestinal tract[17]. There are two types of EC cell: the open type and the closed type. Open-type EC have a large basolateral compartment in contact with the basal lamina, and a narrow apical process that allows access to the lumen. The present study demonstrated that at least some of the ANP-synthesizing cells were exposed to both basal lamina and lumen in the gastric mucosa, and, by using immuno-electron microscopy, they were identified as EC cells that synthesized ANP. Immunoreactive ANP has been reported in immune-type cells in lymphatic nodules in the lamina propria and the submucosa in guinea pig and rat intestine[18]. However, in the human stomach immunoreactive ANP has been not found in the lamina propria or submucosa[19,20]. In the present study, no staining for ANP-synthesizing cell was detected in the lamina propria, submucosa or smooth muscle. However, there were some microvessels scattered in the antral mucosa, and some branch arteries from the large vessels ran into the basal glands of the gastric mucosa. The density of microvessels was different in different positions: in the areas where more basal glands were distributed, more microvessels were found in the gastric mucosa. There was a significant positive relationship between EC cell density and microvessel density in the antral mucosa of rats. However, there was a negative relationship between density of micro-vessels and ANP-synthesizing cells in the gastric fundus and body mucosa. These results suggest that the basolateral plasma membrane of EC cell in antral gastric mucosa may be adjacent to microvessels by which ANP could enter the circulation from EC cell. Because the EC cells in the gastric mucosa are open-type endocrine cells, they can receive chemical stimulation from the gastric lumen and also from microvessels, by which process ANP secretion can be regul-ated. ANP generated by EC cells may enter the circulation from the gastric mucosa and regulate gastric motility via NPR in smooth muscles.
Our results are in agreement with and extend the observations of Li and Goy[21] and Rambotti et al[22], who demonstrated the presence of NPR-A and NPR-B transcripts in extracts of gastric fundus, and localized natriuretic peptide-induced cGMP production to parietal cells, mucus secreting cells in the fundus, and pyloric glands, as well as gastric smooth muscle cells. ANP is known to stimulate gastric acid secretion and relax gastric smooth muscle[10,18]. Further evidence for the existence of functional receptors in gastric tissues comes from reports that ANP stimulates the production of cGMP in guinea pig chief cells, and that ANP induces the relaxation of cultured gastric smooth muscle cells[23,24]. ANP released locally into the gastric lumen could target these luminally directed receptors, according to the finding by Rambotti et al[22] that there is ANP-induced guanylate cyclase activity in both the apical and basolateral surfaces of mucosal cells within the pyloric glands of rat stomach. This suggests that ANP may help control a “negative feedback” system within the stomach, whereby mucus production is enhanced in response to increasing acid secretion to protect the lining of the stomach from the effects of acid. This would provide a regulatory mechanism to ensure that the acid produced after a meal does not injure the mucosal surface of the stomach.
In conclusion, our results demonstrated that EC cells synthesized ANP in rat gastric mucosa, and that the density of ANP-synthesizing cells in the gastric mucosa was greatest in the gastric fundus, intermediate in the antrum, and the lowest in the body. Because there is a positive relationship between ANP-synthesizing cells and microvessel density only in the gastric antral mucosa, ANP may regulate gastric acid and mucus secretion by paracrine methods and gastric motility by endocrine methods; that is, ANP may be transported from EC cells to smooth muscle through microvessels. EC cells may receive chemical stimulation from the gastric lumen and also from microvessels, by which methods ANP secretion could be regulated.
References
- De Bold AJ, Borenstein HB, Veress AT, Sonnenbery H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extracts in rats. J Am Soc Nephrol 2001;12:403-9.
- Lai FJ, Hsieh MC, Hsin SC, Lin SR, Guh JY, Chen HC, et al. The cellular localization of increased atrial natriuretic peptide mRNA and immunoreactivity in diabetic rat kidneys. J Histochem Cytochem 2002;50:1501-8.
- Cayli S, Ustunel I, Celik-Ozenci C, Korgun ET, Demir R. Distribution patterns of PCNA and ANP in perinatal stages of the developing rat heart. Acta Histochem 2002;104:271-7.
- Zhao L, Mason NA, Strange JW, Walker H, Wilkins MR. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic peptide activity. Circulation 2003;107:234-7.
- Zhao L, Mason NA, Strange JW, Walker H, Wilkins MR. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic peptide activity. Circulation 2003;107:234-7.
- Peng N, Chambless BD, Oparil S, Wyss JM. Alpha2A-adrenergic receptors mediate sympathoinhibitory responses to atrial natriuretic peptide in the mouse anterior hypothalamic nucleus. Hypertension 2003;41:571-5.
- Gower WR Jr, Salhab KF, Foulis WL, Pillai N, Bundy JR, Vesely DL, et al. Regulation of atrial natriuretic peptide gene expression in gastric antrum by fasting. Am J Physiol Regul Integr Comp Physiol 2000;278:R770-80.
- Gower WR, McCuen RW, Arimura A, Coy DA, Dietz JR, Landon CS, et al. Reciprocal paracrine pathways link atrial natriuretic peptide and somatostatin secretion in the antrum of the stomach. Regul Pept 2003;110:101-6.
- Gerbes AL, Nathrat W, Cantin M, Denecke H. Presence of atrial natriuretic factor prohormone in enterochromaffin cells of the human large intestine. Gastroenterology 1991;101:424-9.
- Guo HS, Xun C, Cui YG, Kim SZ, Cho KW, Li ZL, et al. Inhibitory effect of C-type natriuretic peptide on spontaneous contraction in antral circular smooth muscle of rat. Acta Pharmacol Sin 2003;24:1021-6.
- Guo HS, Jin Z, Jin ZY, Li ZH, Cui YF, Wang ZY, et al. Comparative study in the effect of C-type natriuretic peptide on gastric motility in various animals. World J Gastroenterol 2003;9:547-52.
- Grube D. The endocrine cells of the digestive system: amine, peptides, and modes of action. Anat Embryol 1986;175:151-62.
- Kong XY, Zhao SM, Yang FG, Ma Q, Zhao LX, Liu S, et al. Quantitative investigations of microvessels by TA-Fe method in different regions of crerebrum. Prog Anat Sci 2004;10:100-2. Chinese..
- Ma Q, Zhao SM, Kong XY, Rong Y, Liu S. Observation of pulmonary microvasculature and cells with tannic acid-ferric chloride (TA-Fe) staining under the light microscope. Prog Anat Sci 2003;9:230-2. Chinese..
- Osman AH, Yuge S, Hyodo S, Sato S, Maeda S, Marie H, et al. Molecular identification and immunohistochemical localization of atrial natriuretic peptide in the heart of the dromedary camel (Camelus dromedaries). Comp Biochem Physiol A Mol Integr Physiol 2004;139:471-4.
- Gower WR Jr, Dietz JR, Vesely DL, Finley CL, Skolnick KA, Fabri PJ, et al. Atrial natriuretic peptide gene expression in the rat gastrointestinal tract. Biochem Biophys Res Commun 1994;202:562-70.
- Vuolteenaho O, Arjama O, Vakkuri O, Maksniemi T, Nikkila L, Kangas J, et al. Atrial natriuretic peptide (ANP) in the rat gastrointestinal tract. FEBS Lett 1988;233:79-82.
- Sundler F, Bottcher G, Ekblad E, Hakanson R. The neuroendocrine system of the gut. Acta Oncol 1989;28:303-14.
- Gower WR Jr, Skvorak JP. The gastrointestinal natriuretic peptide system: a regional system with tissue-specific and vascular components. In: Vesely DL, editor. Atrial natriuretic peptides. Trivandrum, India: Research Signpost; 1997. p 139–50.
- Ehrenreich H, Sinowatz F, Schulz R, Arendt RM, Goebel FD. Immunoreactive atrial natriuretic peptide (ANP) in endoscopic biopsies of human gastrointestinal tract. Res Exp Med 1989;189:421-5.
- Li Z, Goy MF. Peptide-regulated guanylate cyclase pathways in rat colon: in situ localization of GCA, GCC, and guanylin mRNA. Am J Physiol Gastrointest Liver Physiol 1993;265:G394-402.
- Rambotti MG, Giambanco I, Spreca A. Detection of guanylate cyclases A and B stimulated by natriuretic peptides in gastrointestinal tract of rat. Histochem J 1996;29:117-26.
- Cherner JA, Singh G, Naik L. Atrial natriuretic factor activates membrane-bound guanylate cyclase of chief cells. Life Sci 1990;47:669-77.
- Chijiiwa Y, Kabemura T, Misawa T, Kawakami O, Nawata H. Direct inhibitory effect of calcitonin gene-related peptide and atrial natriuretic peptide on gastric smooth muscle cells via different mechanisms. Life Sci 1992;50:1615-23.
