Effects of neutral sulfate berberine on LPS-induced cardiomyocyte TNF-α secretion, abnormal calcium cycling, and cardiac dysfunction in rats1
Introduction
Myocardial depression is a common complication in patients with septic shock, and 40% of patients with sepsis develop cardiac dysfunction characterized by biventricular dilatation, decreased ejection fraction, reduced response to fluid resuscitation, and altered cardiac index[1]. Parker et al reported that these cardiovascular abnormalities were reversible in surviving patients, but not in non-survivors, demonstrating that myocardial depression significantly contributed to the mortality of patients with septic shock[2]. It is generally believed that endotoxin, a lipopolysaccharide (LPS) component of the outer membrane of Gram-negative bacteria, was a major mediator of the cardiovascular dysfunction during sepsis. Administration of LPS to healthy humans could induce myocardial dysfunction, a phenomenon that has been simulated in experimental animal models[3,4]. Evidently, it was very important to develop a potential therapeutic agent for LPS-induced heart failure.
Berberine, an isoquinoline derivative alkaloid, is a major component of Rhizoma coptidis (Huanglian), a herb used in traditional Chinese medicine. Berberine has been found to have multiple pharmacological activities, including antidiarrheal, anti-tumor and anti-inflammatory effects, and vasorelaxant and antiproliferative properties[5–7]. Some studies have demonstrated that berberine has wide cardiovascular effects. Chiou et al reported that berberine induced endothelium-independent vascular relaxation via blocking the release of Ca2+ from internal stores[8]. Recently, the efficacy and safety of berberine for the treatment of chronic heart failure was assessed, and it was demonstrated that treatment with berberine increased left ventricular ejection fraction and exercise capacity, decreased the frequency and complexity of ventricular premature complexes, improved quality of life and decreased mortality in patients with congestive heart failure[9]. However, it is still unclear whether berberine has a prophylactic or therapeutic effect on LPS-induced myocardial dysfunction.
Although the mechanism of LPS-induced myocardial dysfunction is not completely understood, it has recently been established that upregulation of tumor necrosis factor α (TNF-α) levels and abnormal calcium cycling in cardiomyo-cytes were involved in LPS-induced myocardial dysfunction[10]. Therefore, in the present study we examined the effect of neutral sulfate berberine on TNF-α release from and intracellular calcium concentration ([Ca2+]i) in cardiomyocytes exposed to LPS. In order to verify the direct action of neutral sulfate berberine on LPS-induced cardiac dysfunction, changes in cardiac function of isolated intact rat hearts exposed to LPS and/or neutral sulfate berberine were also determined.
Materials and methods
Cell culture preparation Neonatal Sprague-Dawley rats were purchased from the Guangdong Province Center for Laboratory Animals. All animals received humane care. Primary cultured rat cardiomyocytes were prepared from the ventricles of 3–4-day-old Sprague-Dawley rats according to the published procedure[11]. Briefly, the neonatal rats were killed and their hearts were excised. The ventricles were minced and digested in a 0.125% trypsin solution [0.2 g/L ethylenediamine tetraacetic acid (EDTA), Ca2+/Mg2+-free Hanks’ buffered salt solution]. Then the cells were collected and resuspended in Dulbecco’s modified Eagles’ medium (DMEM)-F12 (Gibco-BRL) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin. After 2-h incubation at 37 °C in 5% carbon dioxide and 95% oxygen in a humidified incubator (Thermo Forma, USA), most of the contaminating fibroblasts were attached to the culture flask, and the cardiomyocytes were prepared from the unattached cells. Cardiomyocyte viability was examined using trypan blue exclusion, and the cell suspension was adjusted to a density of 5×105 cells/mL and cultured for 48 h, during which time the attached cardiomyocytes began to contract spontaneously. The cells were used in the experiments on d 4 of culture.
Measurement of TNF-α Neonatal cardiomyocytes plated at a density of 5×105 cells/mL were incubated with LPS (O55:B5, Sigma) at concentrations of 1, 5, 10, and 20 µg/mL for 6 h. In another experiment, the neonatal rat cardiomyocytes were treated with 10 µg/mL LPS alone or in combination with 1, 2, or 4 µmol/L neutral sulfate berberine (Sigma) for 6 h. TNF-α concentrations in cell-conditioned media were measured by using a Quantikine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, USA) for rat TNF-α, according to the manufacturer’s instructions.
Fura-2 acetoxymethyl ester loading and measurement of [Ca2+]i Neonatal rat cardiomyocytes (5×105 cells/mL) were loaded with Fura-2/acetoxymethyl (AM) ester (Sigma) at a final concentration of 5 µmol/L for 30 min in the dark, then the cells were washed with Hanks’ solution 3 times to remove the extracellular Fura-2/AM. Based on methods described previously[12], Fura-2 fluorescence was monitored with a Shimadzu RF-5000 fluorescence spectrophotometer (Japan) with excitation at 340 and 380 nm and emission at 510 nm, which was performed in a water-jacketed cuvette at 37 °C. Basal and agent-induced changes in the excitation ratio of Fura-2 (340/380 nm) were measured for approximately 10 min after the addition of 50 µmol/mL KCl, 5 µg/mL isoproterenol, 200 µg/mL LPS and 1 or 2 nmol/mL neutral sulfate berberine. Maximum (Rmax) and minimum (Rmin) fluorescence values were determined by adding 50 µL of Triton X-100 (10%) and 100 µmol/L ethyleneglycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), respectively. The cardiomyocyte [Ca2+]i was then calculated. In some experiments, the neonatal rat cardiomyocytes were treated with 200 μg/mL LPS and/or 2 nmol/mL neutral sulfate berberine for 1–2 h, after which the cardiomyocyte [Ca2+]i was examined using Fura-2/AM.
Perfused heart preparation Based on a method described by Stamm et al[10], isolated rat hearts were perfused in Langendorff mode. In brief, male Sprague-Dawley rats weighing 200±20 g, obtained from the Guangdong Province Center for Laboratory Animals were anticoagulated with heparin (200 U, iv) and anesthetized with intraperitoneal administration of sodium pentobarbital (35 mg/kg). The hearts were rapidly excised and arrested in chilled normal saline. After the aorta was cannulated, hearts were perfused retrogradely on a Langendorff apparatus with filtered Krebs-Henseleit buffer at 37 °C and a constant pressure of 80 cmH2O. The Krebs-Henseleit solution was composed of (mmol/L): NaCl 118, KCl 4.7, KH2PO4 1.2, NaHCO3 25, CaCl2 2.5, MgSO4 1.2, and glucose 11. The solution was equilibrated with a 5% CO2 and 95% O2 gas mixture (pH 7.35–7.45). A small latex balloon filled with fluid was inserted in the left ventricle and connected to a pressure transducer, then the maximal rate of left ventricular pressure rise and fall (±dp/dtmax) and heart rate were measured by using a Biolab-410 physiological function system (China) throughout the experiment. The hearts were divided into 4 groups: the hearts in the control group were perfused with Krebs-Henseleit buffer; the hearts in the LPS group were perfused with 100 µg/mL LPS for 20 min followed by Krebs-Henseleit buffer; in the neutral sulfate berberine plus LPS group, the hearts were infused with 1 µmol/L berberine for 10 min followed by 100 μg/mL LPS for 20 min, and Krebs-Henseleit buffer to the end of the experiment; and the hearts in the berberine group were perfused with 1 µmol/L berberine for 10 min followed by Krebs-Henseleit buffer to the end of the experiment.
Statistical analysis Results are expressed as mean±SD. Statistical analysis was performed using the SPSS software package (version 8.0). Paired t-tests, Student’s t-test and one-way ANOVA followed by the Student-Newman-Keuls test were used to determine statistical significance. The difference between means was considered statistically significant when P was less than 0.05.
Results
Influence of LPS and neutral sulfate berberine on TNF-α secretion in neonatal rat cardiomyocytes LPS stimulated TNF-α release from cardiomyocytes. In the presence of 10% fetal bovine serum, untreated cardiomyocytes produced a basal level of 86.6±7.3 pg/mL of TNF-α. Treatment of cardio-myocytes for 6 h with LPS at concentrations of 1, 5, 10, and 20 μg/mL all caused a significant increase in the amount of TNF-α released (Figure 1). In contrast, berberine inhibited LPS-induced TNF-α release from neonatal rat cardiomyo-cytes in a dose-dependent manner. Berberine at a concentration of 4 nmol/mL almost completely prevented the increase in TNF-α release in cardiomyocytes exposed to 10 μg/mL LPS (Table 1).
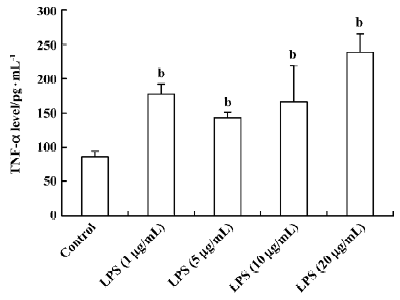
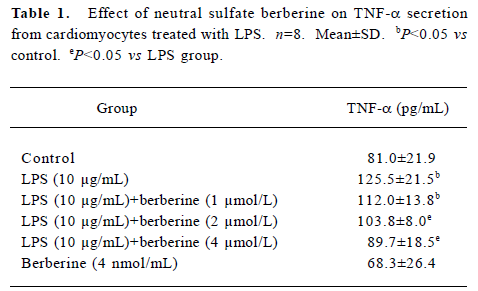
Full table
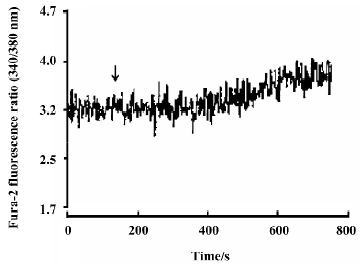
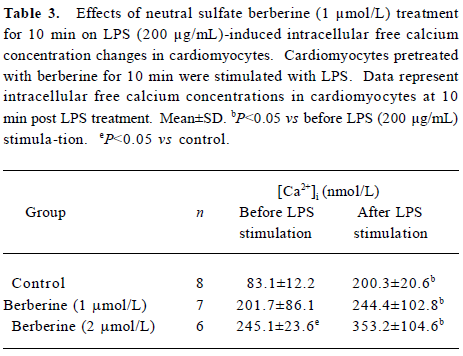
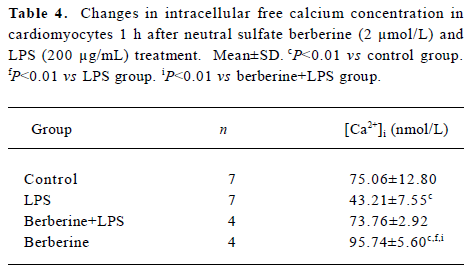
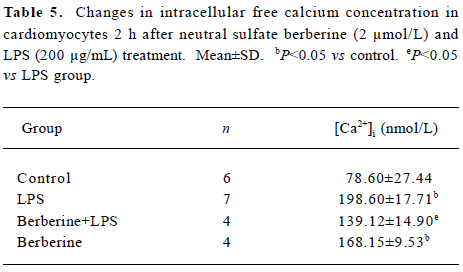
Effects of neutral sulfate berberine on [Ca2+]i changes in neonatal rat cardiomyocytes exposed to KCl, isopro-terenol, and LPS Addition of 50 mmol/L KCl caused an increase in the [Ca2+]i of cardiomyocytes from 70.61±4.26 nmol/L to 159.63±11.88 nmol/L (n=6, P<0.01), and this increase was completely suppressed by pretreatment of the cardiomyocytes with 2 µmol/L berberine (75.26±6.06 nmol/L; Figure 2). As shown in Table 2, pretreatment of cardio-myocytes with 2 µmol/L berberine for 10 min did not suppress the isoproterenol-evoked increase in [Ca2+]i of cardio-myocytes. LPS at a concentration of 200 µg/mL evoked a rapid increase in [Ca2+]i of cardiomyocytes 10 min after stimulation (Figure 3). Berberine treatment alone for 10 min stimulated an increase in the [Ca2+]i of cardiomyocytes, but did not inhibit the LPS-induced rapid elevation of the [Ca2+]i of cardiomyocytes (Table 3). However, [Ca2+]i in cardiomyo-cytes decreased markedly when the cells were treated with LPS (200 µg/mL) for 1 h, and became significantly elevated 2 h after LPS exposure. Both of these effects were reversed by 2 µmol/L berberine. Berberine treatment for 1 and 2 h pro-duced an elevation in [Ca2+]i of cardiomyocytes (Table 4, 5).
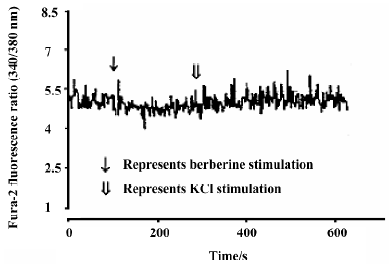
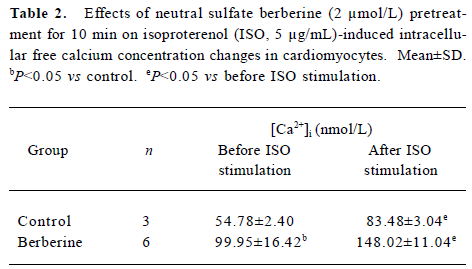
Full table
Full table
Full table
Full table
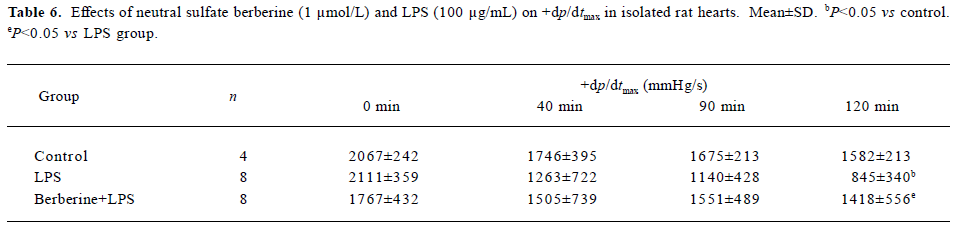
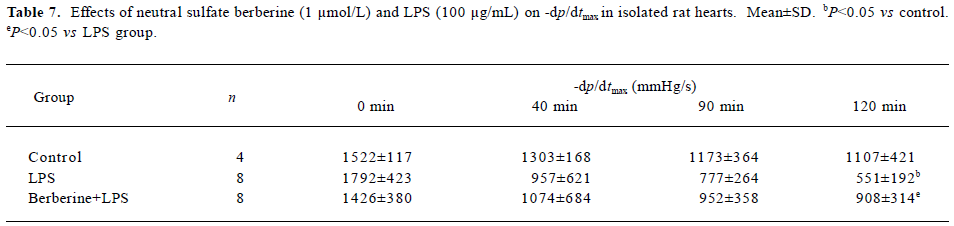
Effects of neutral sulfate berberine and LPS on heart rate and ±dp/dtmax in isolated rat hearts Infusion with 100 µg/mL LPS for 20 min significantly decreased the heart rate from 256±56 beats/min to 140±104 beats/min at 120 min in isolated rat hearts (P<0.05), whereas this effect was not found in control hearts. Infusion with 1 µmol/L berberine for 10 min also reduced the heart rate from 289±35 beats/min to 194±38 beats/min at 120 min (P<0.05). Moreover, berberine did not antagonize the LPS-induced drop in heart rate (from 253±56 beats/min to 160 ± 49 beats/min at 120 min). As shown in Tables 6 and 7, perfusion of isolated heart with 100 µg/mL LPS for 20 min resulted in significantly impaired cardiac performance at 120 min after LPS challenge, and the maximal rate of left ventricular pressure rise and fall (±dp/dtmax) decreased compared with the controls. In contrast, ±dp/dtmax at 120 min of hearts perfused with 1 µ mol/L berberine for 10 min, followed by 100 µg/mL LPS for 20 min was higher than that for hearts in the LPS group.
Full table
Full table
Discussion
The mechanism of LPS-induced cardiodepression remains controversial. Recently, some evidence has demonstrated that TNF-α contributes to LPS-induced cardiac dysfunction, although other mediators have been involved in this process[10,13]. Kapadia et al reported that during endotoxemia, TNF-α gene and protein expression increased in feline myocardium[14]. Furthermore, LPS has been found to induce the secretion of TNF-α from cultured adult rat cardiomyocytes via the CD14 signal pathway, LPS has also been shown to trigger apoptosis in rat cardiomyocytes, which can be blocked by pretreatment with the soluble TNF-α fragment, indicating that the cardiodepressant action of LPS may be in part due to TNF-α-induced apoptosis, which decreases the number of working myocardial cells[15]. Inhibition of myocardial NF-κB activation in transgenic mice constitutively overexpressing a nondegradable I-κBα in cardiomyocytes can inhibit cardiac TNF-α synthesis and prevent cardiac dysfunction induced by LPS[4]. In the present study, we confirmed that LPS stimulated TNF-α secretion from neonatal rat cardiomyocytes and, importantly, we also found that neutral sulfate berberine dose-dependently inhibited TNF-α release from cardiomyocytes exposed to LPS, although the mechanism by which neutral sulfate berberine suppresses LPS-stimulated TNF-α release from cardio-myocytes remains to be further investigated.
There is some evidence that abnormal calcium cycling in cardiomyocytes may be related to LPS-induced cardiac depression, although reports from different laboratories are inconsistent[16–18]. Stamm and colleagues demonstrated that recirculating perfusion of isolated rat heart with LPS for 30 min significantly impaired myocardial contractility, which was associated with lower [Ca2+]i levels and attenuated systolic increases in [Ca2+]i. They found that these LPS effects were not only mimicked by rat TNF-α but also blocked by anti-TNF-α antibody, suggesting a direct relationship between LPS-induced TNF-α release, abnormal calcium cycling and reduced contractility in intact hearts[10]. Thompson et al reported that 18-h exposure to LPS stimulated myocardial calcium overload and that dantrolene inhibited calcium overload and improved LPS-induced cardiac dysfunction[19]. Therefore, decreases in [Ca2+] i in the early stage and calcium overload in the later stage after LPS challenge played important roles in LPS-induced myocardial depression. In the present study, we observed that neutral sulfate berberine blocked a KCl-evoked increase in [Ca2+]i, although neutral sulfate berberine itself induced a rapid rise in [Ca2+]i in cardiomyocytes. However, neutral sulfate berberine could not inhibit isoproterenol- and LPS-evoked rapid increases in cardiomyocyte [Ca2+]i. These data indicated that LPS-stimulated rapid increases in [Ca2+]i might involve multiple mechanisms, including calcium release from the sarcoplasmic reticulum. However, [Ca2+]i in cardiomyocytes decreased markedly when the cells were treated with LPS for 1 h, and elevated significantly 2 h after LPS exposure, both of which were reversed by neutral sulfate berberine.
In summary, we observed that neutral sulfate berberine not only inhibited TNF-α release, but also prevented abnormal calcium cycling in cardiomyocytes, suggesting that neutral sulfate berberine may improve LPS-induced cardiac dysfunction. To further test this hypothesis, we also examined the effect of neutral sulfate berberine on LPS-induced myocardial depression in isolated intact rat hearts. We found that neutral sulfate berberine itself had a negative effect on frequency in isolated whole rat hearts, and did not reverse the LPS-induced negative impact on frequency, but prevented LPS-induced systolic and diastolic ventricular dysfunction. These findings demonstrated that neutral sulfate berberine inhibited LPS-stimulated TNF-α release and normalized intracellular calcium levels in cardiomyocytes exposed to LPS, and attenuated LPS-induced myocardial depression. Neutral sulfate berberine may be a potential therapeutic agent for the treatment of LPS-induced heart dysfunction, and its in vivo effect on heart function during endotoxemia deserves to be further investigated.
References
- Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 1990;113:227-42.
- Parker MM, McCarthy KE, Ognibene FP, Parrillo JE. Right ventricular dysfunction and dilatation, similar to left ventricular changes, characterize the cardiac depression of septic shock in humans. Chest 1990;97:126-31.
- Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med 1989;321:280-7.
- Haudek SB, Spencer E, Bryant DD, White DJ, Maass D, Horton JW, et al. Overexpression of cardiac I-κBα prevents endotoxin-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol 2001;280:H962-8.
- Zhang MF, Shen YQ. The antidiarrheal and antiinflammatory effects of berberine. Acta Pharmacol Sin 1989;10:174-6.
- Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, et al. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol 2000;399:187-96.
- Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett 2004;203:127-37.
- Chiou WF, Chen J, Chen CF. Relaxation of corpus cavernosum and raised intracavernous pressure by berberine in rabbit. Br J Pharmacol 1998;125:1677-84.
- Zeng XH, Zeng XJ, Li YY. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2003;92:173-6.
- Stamm C, Cowan DB, Friehs I, Noria S, del Nido PJ, McGwan FX Jr. Rapid endotoxin-induced alterations in myocardial calcium handling: obligatory role of cardiac TNF-α. Anesthesiology 2001;95:1396-405.
- Liao XD, Tang AH, Chen Q, Jin HJ, Wu CH, Chen LY, et al. Role of Ca2+ signaling in initiation of stretch-induced apoptosis in neonatal heart cells. Biochem Biophys Res Commun 2003;310:405-11.
- Liu PG, Xu YJ, Hopfner RL, Gopalakrishnan V. Phosphatidic acid increases inositol-1,4,5-trisphosphate and [Ca2+]i levels in neonatal rat cardiomyocytes. Biochim Biophys Acta 1999;1440:89-99.
- Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor-alpha and interleukin 1-beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 1996;183:949-58.
- Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-α gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest 1995;96:1042-52.
- Comstock KL, Krown KA, Page MT, Martin D, Ho P, Pedraza M, et al. LPS-induced TNF-α release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol 1998;30:2761-75.
- Rigby SL, Hofmann PA, Zhong J, Adams HR, Rubin LJ. Endotoxemia-induced myocardial dysfunction is not associated with changes in myofilament Ca2+ responsiveness. Am J Physiol 1998;274:H580-90.
- Tavernier B, Mebazaa A, Mateo P, Sys S, Ventura-Clapier R, Veksler V. Phosphorylation-dependent alteration in myofilament Ca2+ sensitivity but normal mitochondrial function in septic heart. Am J Respir Crit Care Med 2001;163:362-7.
- Ziolo MT, Katoh H, Bers DM. Expression of inducible nitric oxide synthase depresses beta-adrenergic-stimulated calcium release from the sarcoplasmic reticulum in intact ventricular myocytes. Circulation 2001;104:2961-6.
- Thompson M, Kliewer A, Maass D, Becker L, White DJ, Bryant D, et al. Increased cardiomyocyte intracellular calcium during endotoxin-induced cardiac dysfunction in guinea pigs. Pediatr Res 2000;47:669-76.
