Baculovirus ETL promoter acts as a shuttle promoter between insect cells and mammalian cells1
Introduction
The baculovirus-insect expression system is recognized as an excellent tool for the production of recombinant proteins[1,2]. Since Smith et al successfully used the Autographa californica multiple nucleopolyhedrovirus (AcMNPV) as an expression vector to produce human β-interferon in insect cells in 1983[3], hundreds of foreign genes have been expressed by using baculovirus expression vector systems (BEVS) to date[4]. When placed under the control of the baculovirus polyhedrin promoter (polh promoter) and by replacing the viral polyhedrin gene, recombinant proteins can be expressed at high levels in infected insect cells and larvae, typically at levels ranging between 30% and 50% of the total insect cell proteins during the very late stages of infection[5].
Baculovirus host specificity has long been considered to be restricted to cells derived from arthropods. However, Hofmann et al first demonstrated that baculovirus-derived vectors were able to drive the expression of a reporter gene, the luciferase gene, in human hepatocytes, provided that a cytomegalovirus (CMV) promoter instead of the polh promoter controls transgene expression[6]. Boyce and Bucher also showed that a human liver tumor cell line, HepG2, and primary rat hepatocytes were able to efficiently express a different reporter gene, the lacZ gene, under the control of the Rous sarcoma virus (RSV) promoter[7]. In both of these pioneering studies, high levels of expression were achieved only in hepatocytes, but only low levels of reporter gene expression were found in other cell lines, such as 293, COS-1, and T-47D. Shoji et al subsequently demonstrated that a chimeric CAG promoter, containing the CMV IE enhancer, chicken β-actin promoter, and rabbit β-globin polyadenyla-tion signal, can expand gene expression in non-hepatic cells, such as HeLa and COS-7 cells, by baculovirus transduction[8]. Furthermore, recombinant baculoviruses carrying a reporter gene under the control of the CAG promoter have been reported to efficiently transduce neural cells in vitro and in vivo[9]. Thus, a baculovirus containing a mammalian promoter element is also a promising tool for gene transfer into mammalian cells in order to study the function of foreign genes, to develop gene therapy strategies, or to express proteins in mammalian cells.
Traditional mammalian promoters, such the simian virus 40 (SV 40) and cytomegalovirus early promoters, do not function well in insect cell systems[10,11]. However, the AcMNPV transactivator IE1 is functional in mammalian cells[12]. Because IE1 is able to activate several early, late and very late promoters in the AcMNPV genome, it would be interesting to test whether the baculovirus promoters, including the IE1 promoter, are functional in mammalian cells. Previous studies have indicated that the AcMNPV early to late promoter (ETL promoter) can mediate reporter gene expression in AcMNPV nonpermissive insect cell lines that do not support the virus’s replication[13]. The ETL gene product, proliferating cell nuclear antigen (PCNA), is a protein with a molecular mass of 28 kDa[14]. In the present study, we demonstrate that the baculovirus ETL promoter can act as a shuttle promoter between insect cells and mammalian cells. We found that ETL promoter activity in mammalian cells was dependent on baculovirus gene expression and that ETL promoter activity was higher in hFob1.19 bone-derived cells than in COS1, HeLa, CHO-K1, and MCF-7 mammalian cells. These results will facilitate the development of baculovirus vector-based mammalian cell gene delivery systems.
Materials and methods
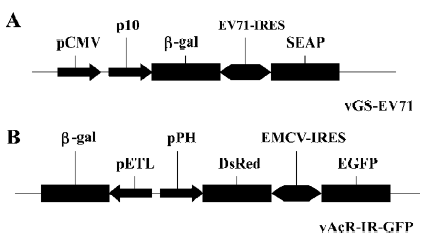
Cells, plasmids and viruses The Spodoptera frugiperda IPBL-Sf21 (Sf21) cell line was cultured in TNM-FH insect medium containing 8% heat-inactivated fetal bovine serum[15]. The recombinant viruses used in the present study were vGS-EV71 and vAcR-IR-G. vGS-EV71 was generated by cotransfection of linearized viral DNA, Bakpak6 (Clontech, Mountain View, CA USA), with transfer vector pGS-EV71 in SF21 cells. pGS-EV71 contains the internal ribosomal entry site (IRES) element derived from enterovirus 71 (EV71) flanked by the β-galactosidase and secreted alkaline phosphatase (SEAP) reporter genes[16] (Figure 1A). vAcR-IR-G was generated by cotransfection of linearized viral DNA, Bac-N-Blue (Invitrogen), with transfer vector pBacDIRE in SF21 cells. pBacDIRE contains the IRES element of the encephalomyo-carditis virus (EMCV) flanked by the red fluorescent protein (DsRed) and enhanced green fluorescent protein (EGFP) genes[17] (Figure 1B). vAcR-IR-G also contains the β-galactosidase gene under the control of the ETL promoter (Figure 1). Sf21 monolayers were used for virus propagation, and all viral stocks were prepared and titers determined according to the standard protocols described by O’Reilly et al[1]. All mammalian cell lines (African green monkey kidney fibroblast-like cell line, COS1; human breast cancer cell line, MCF-7; human cervix carcinoma cell line, HeLa; Chinese hamster ovary cells, CHO-K1; and human fetal osteoblastic cells, hFob1.19) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Sigma) containing 10% fetal bovine serum. Sodium pyruvate containing 1 and 0.1 mmol/L non-essential amino acids were added to the culture medium of MCF-7.
Infection of insect cells and transduction of mammalian cells Cells were seeded in 24-well plates at 1×105–2×105 cells/well. Culture medium was removed and replaced with virus inocula, and the mixture was centrifuged at 2000 rpm for 1 h. Then the supernatant was removed, and fresh medium was added, and the cells were cultured at 27 ºC (for insect cells) or 37 °C (for mammalian cells). Forty-eight hours after transduction or infection, the reporter gene assays were carried out.
Transfection of mammalian cells Transfections of mammalian cells were performed using the Lipofectin reagent (Invitrogen). Cells (1×105–2×105) were plated onto 24-well plates. Before transfection, cells were repeatedly washed with serum-free media to remove all traces of sera. One microgram of plasmid was diluted in 200 µL of serum-free medium, and 2 µL of Lipofectin reagent was added. The DNA-Lipofectin mix was incubated for 15 min for DNA-Lipofectin complex formation. Then, the DNA–Lipofectin complex solution was transferred to the cells, and serum-free medium was added to adjust the total volume to 0.5 mL. After 12 h, the medium was removed, and 1 mL of fresh medium with 10% fetal bovine serum was added.
β-Galactosidase assay Cells (1×105 per well) were infected with vAcR-IR-G or vGSEV-71 at multiplicity of infection (MOI) values of 10 or 100, respectively. Cell extracts were prepared 48 h after infection. Levels of β-galactosidase activity were measured using a Luminescent β-galactosidase Detection Kit II (BD Biosciences) with a Luminator (Mithras LB940; Berthold Technologies). β-Galactosidase activity was expressed as relative light units (RLU).
For in vitro staining of β-galactosidase activity, cells were washed twice with phosphate-buffered saline (PBS) and fixed with 0.25% glutaraldehyde at 48 h post-infection or transduction. After they were rinsed twice with PBS, cells were stained with a solution containing 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal), 5 mmol/L potassium ferrocyanide, 5 mmol/L potassium hexacyano-ferrate, and 2 mmol/L MgCl2 in PBS.
Results
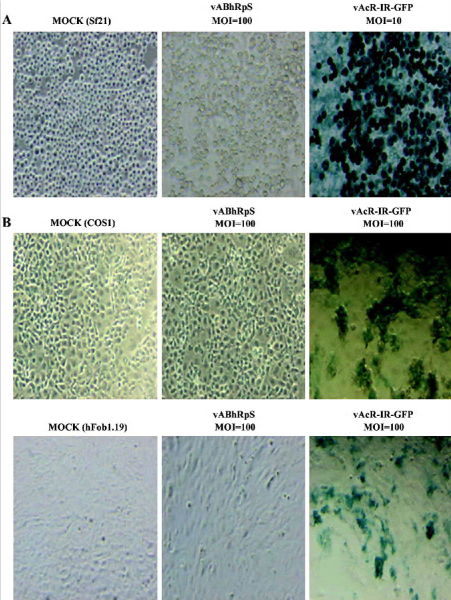
ETL promoter of AcMNPV is a shuttle promoter Because the bicistronic plasmid pGS-EV71 was based on pTriEX-4 (Norvegon, Madison, WI), a baculovirus transfer vector[16], we were able to generate the recombinant virus, vGS-EV71 (Figure 1A). vGS-EV71 contained the β-galactosidase and SEAP reporter genes under the control of the baculovirus p10 promoter and CMV promoter, so we tried to monitor the expression of β-galactosidase and SEAP in vGS-EV71-infected insect Sf21 cells and in vGS-EV71-transduced COS-1 cells to test whether the EV71-IRES could still mediate cap-independent function in the baculovirus genome. In this experiment, we used vAcR-IR-G (Figure 1B), which contained the green and red fluorescence genes flanking the EMCV IRES sequence under the polh promoter, as the control. vAcR-IR-G-infected Sf21 cells appeared blue after X-Gal staining as expected (Figure 2A), because the baculovirus ETL promoter can function well in Sf21 cells. However, the COS1 cells transduced with vAcR-IR-G were also blue after X-Gal staining, which was unexpected (Figure 2B), and vAcR-IR-G-infected hFob1.19 cells also appeared blue after X-Gal staining. To rule out the possibility that the X-Gal staining signal stemmed from baculovirus infection, we infected the Sf21 cells and transduced the COS1 and hFob1.19 mammalian cells with a β-galactosidase gene-deficient recombinant virus, vABhRps, which contained the red fluorescent protein gene under the control of a heat shock promoter. Figure 2A and 2B show that neither the vABhRps-infected Sf21 cells nor transduced mammalian cells had blue staining. These results suggest that the baculovirus ETL promoter may act as a shuttle promoter between insect cells and mammalian cells. Furthermore, ETL promoter activity should be immediately early gene-dependent[14]; thus, we hypothesize that COS1 cells and hFob1.19 cells must support baculovirus gene expression at least to the delayed early stage.
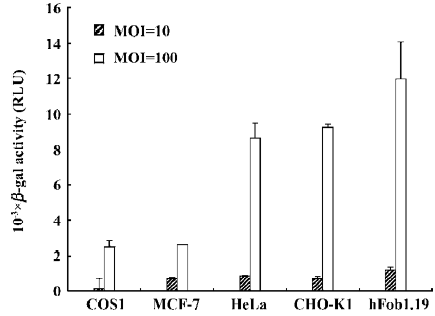
Activity of the ETL promoter in different mammalian cells To analyze the cell tropism of the ETL promoter, we tried to transduce COS1, HeLa, CHO-K1, hFob1.19, and MCF-7 mammalian cells with vAcR-IR-G, and then quantified ETL promoter activity by using the β-galactosidase activity assay. We detected significant levels of β-galactosidase expression in CHO-K1, hFob1.19, and HeLa cells (Figure 3). The bone-derived cell line hFob1.19 had the highest expression level of β-galactosidase in the tested mammalian cell lines. This result was similar to that noted for the CAG promoter-luciferase gene cassette-based baculovirus transfer vector[8]. However, low-level expression of β-galactosidase was found in COS1 and MCF-7 cells. Figure 3 also shows that all of the tested mammalian cell lines infected with vAcR-IR-G at an MOI of 100 had higher β-galactosidase activity than those infected at an MOI of 10; this is consistent with previous studies, which reported that highly efficient transduction of mammalian cells required baculovirus with a high MOI [6–8].
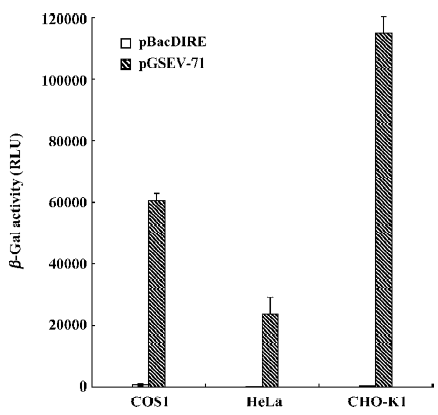
Activity of the ETL promoter in mammalian cells is baculovirus gene expression-dependent To explore whether the baculovirus ETL promoter is transcribed from the mammalian host RNA polymerase or if its transcription is dependent on the baculovirus immediate early gene, we transiently transfected COS-1, HeLa, and CHO-K1 cells with the pBacDIRE and pGSEV-71 plasmids. Figure 4 shows that the CMV promoter in the pGSEV-71 plasmid mediated β-galactosidase gene expression in these cell lines, but that the ETL promoter in pBacDIRE could not mediate β-galactosidase gene expression in mammalian cells. This result implies that ETL promoter activity in baculovirus-infected mammalian cells may be baculovirus early gene (eg IE0, IE1-+, or IE2)-dependent[14]. It will be interesting to test the baculoviral early promoters in mammalian cells, and this result also suggests that early genes and the ETL gene may be transcribed within mammalian cells transduced with recombinant baculoviruses, although the viral DNA did not replicate and no apparent cytopathic effects (CPE) were reported[8].
Butyrate did not enhance the activity of the ETL promoter in mammalian cells Several studies have shown that histone deacetylase (HDAC) inhibitors, such as butyrate, can effectively enhance protein expression in mammalian cells[18,19]. To clarify whether the baculovirus early gene-dependent ETL promoter can also be enhanced by butyrate, we added 10 mmol/L butyrate to the COS-1 cell culture medium after transduction with vAcR-IR-G or vGS-EV71 (Figure 5). We found that the addition of 10 mmol/L butyrate did enhance β-galactosidase gene expression in COS-1 cells transduced with vGS-EV71, with a nearly 10-fold enhancement (Figure 5A). This result is consistent with the results of previous studies, in which butyrate was found to enhance protein expression in baculovirus-transduced mammalian cells[9,20]. However, the addition of 10 mmol/L butyrate did not enhance β-galactosidase gene expression in COS-1 cells transduced with vAcR-IR-G (Figure 5A). We further tested the effect of butyrate on COS-1 cells transduced with vAcR-IR-G at different MOI. Figure 5B shows that the addition of butyrate did not enhance β-galactosidase gene expression controlled by the ETL promoter. Thus, these results revealed that blockage of HDAC activity and then prevention of histone deacetylation could not promote the baculovirus ETL promoter, although blockage of the activity of HDAC often upregulates gene expression.

Discussion
Since recombinant baculoviruses became established as efficient gene transduction vehicles for mammalian cells, recombinant baculoviruses have also become attractive vectors for in vitro and in vivo gene therapy. One of the advantages of using these insect viruses for delivery of therapeutic genes to mammalian cells is that unlike other mammalian viruses, they do not replicate in mammalian cells. Thus, a baculovirus-based vector can be much safer than current viral vectors[21]. In addition, as gene therapy vectors, baculoviruses also possess the following advantages[8,22]: (1) given the flexibility of the baculovirus envelope, large DNA insertions (>20 kb) can be accommodated in the dsDNA genome; (2) recombinant baculoviruses have the ability to reach high titers (>1010 pfu/mL); and (3) recombinant baculoviruses are easily prepared. Recently, studies have demonstrated that baculoviruses can mediate gene transfer into articular chondrocytes[23] and human mesenchymal stem cells[24]. These results will expand the application of baculovirus vector systems into the field of cartilage tissue engineering[22]. In the present study, we demonstrated that the baculovirus ETL promoter could function as a shuttle promoter between insect cells, the hosts of baculoviruses, and mammalian cells (Figures 2 and 3). Neither the traditional CMV promoter nor the CAG promoter appears to function well, if at all, in insect systems[10,11,25]. So the expression of recombinant baculoviruses containing the desired gene under the control of the CMV promoter or CAG promoter cannot be detected until transduction into mammalian cells. In contrast, the desired gene expression under the control of the ETL promoter can be monitored and checked in insect cells before performing mammalian cell transduction experiments. Therefore, the finding that the ETL promoter can act as a shuttle promoter will facilitate the development of baculovirus vector-based mammalian cell gene delivery vehicles.
The susceptibility of various mammalian cell lines to the ETL promoter was monitored by the β-galactosidase reporter assay (Figure 3). We detected significant levels of β-galactosidase expression in CHO-K1, hFob1.19, and HeLa cells. This result was similar to that found for a CAG promoter-luciferase gene cassette-based baculovirus transfer vector[8]. The bone-derived cell line hFob1.19 had the highest expression level of β-galactosidase among the tested mammalian cell lines (Figure 3). Interestingly, previous studies also found that a human bone-derived sarcoma cell line, osteogenic sarcoma cell line (Saos-2), had higher susceptibility than any other known permissive mammalian cells to infection by baculoviruses[21]. However, low-level expression of β-galactosidase was found in COS1 and MCF-7 cells. Previous studies showed that the monkey kidney cell line, like COS1 and COS7, exhibited high-level gene expression through CAG promoter-based baculovirus transfer vectors. So, the transcription factors that are required for the ETL promoter may be restricted in COS1 cells. In addition, we also found that sodium butyrate enhanced CMV promoter-mediated β-galactosidase gene expression, but did not enhance ETL promoter-mediated β-galactosidase gene expression in COS-1 cells (Figure 5). A plasmid-based transient transfection assay also revealed that activation of the ETL promoter in mammalian cells was baculovirus gene expression-dependent (Figure 4). These results indicate that transcription initiation from the ETL promoter differs from that of the CMV promoter.
To study the expression of foreign genes in vitro or in vivo, viral vectors are generally useful. However, the replication or gene expression of viral vectors in the desired host may sometimes cause complex effects. The present study also raised a question about baculovirus gene expression in mammalian cells when recombinant baculoviruses are employed as a gene delivery vehicles. Although previous studies indicated that baculovirus viral DNA would not replicate in mammalian cells, no apparent CPE was observed even when cells were transduced with an MOI as high as 100[8,22]. Our experiments demonstrated that the baculovirus gene will be transcribed by the mammalian RNA polymerase, and that the transcriptional factors required for ETL promoter activity should be activated in mammalian cells. For example, it has been reported that the baculovirus transactivator IE1 is functional in mammalian cells[12]. To investigate the expression profile of AcMNPV genes in mammalian cells, a baculovirus-specific DNA microarray may be critical. Recently, a baculovirus-specific DNA microarray was used to investigate the expression profile of AcMNPV genes in permissive and nonpermissive insect cell lines[26]. Surpri-singly, nearly all of the 154 genes of AcMNPV, including the ETL gene, appeared to be expressed in both permissive (Sf9) and nonpermissive (BmN, a silkworm-derived cell line) cells, although the peak expression levels of these genes were delayed approximately 12 h in nonpermissive BmN cells. Interestingly, the product of the ETL gene is necessary, either directly or indirectly, for the expression of many other AcMNPV genes[14]. So, it is important to determine how many ETL gene-dependent baculovirus intrinsic genes are expressed in mammalian cells before the application of recombinant baculoviruses for gene therapy.
References
- O’Reilly DR, Miller LK, Luckow VA. Cotransfection and recombinant virus identification. In: O’Reilly DR., Miller LK, Luckow VA, editors. Baculovirus expression vector: a laboratory manual. New York: WH Freeman; 1992. p139–66.
- Galleno M, Sick AJ. Baculovirus expression vector system. In: Fernandez JM, Hoeffler JP, editors. Gene expression systems: using nature for the art of expression. San Diego: Academic Press; 1999. p332–59.
- Smith GE, Summers MD, Fraser MJ. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol 1983;3:2156-65.
- Possee RD. Baculoviruses as expression vectors. Curr Opin Biotechnol 1997;8:569-72.
- Cha HJ, Dalal NG, Pham MQ, Kramer SF, Vakharia VN, Bentley WE. Monitoring foreign protein expression under baculovirus p10 and polh promoters in insect larvae. BioTechniques 2002;32:986-92.
- Hofman C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA 1995;92:10099-03.
- Boyce FM, Bucher NL. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA 1996;93:2348-52.
- Shoji I, Aizaki H, Tani H, Ishii K, Chiba T, Saito I, et al. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J Gen Virol 1997;78:2657-64.
- Sarkis C, Serguera C, Petres S, Buchet D, Ridet JL, Edelman L, et al. Efficient transduction of neural cells in vitro and in vivo by a baculovirus-derived vector. Proc Natl Acad Sci USA 2000;97:14638-43.
- Pfiefer TA, Hegedus DD, Theilmann DA, Grigliatti TA. Baculovirus immediate-early promoter-mediated expression of the ZeocinTM resistance gene for use as a dominant selectable marker in dipteran and lepidoteran insect cell lines. Gene 1997;188:183-90.
- Bourouis M, Jarry B. Vectors containing a prokaryotic dihydrofolate reductase gene transform Drosophila cells to methotrexate-resistance. EMBO J 1983;2:1099-104.
- Daniela M, Andreas K, Dagmar KM. Baculovirus transactivator IE1 is functional in mammalian cells. J Gen Virol 1997;78:1507-10.
- Morris TD, Miller LK. Promoter influence on baculovirus mediated gene expression in permissive and non-permissive insect cell lines. J Virol 1992;66:7397-405.
- Crawford AM, Miller LK. Characterization of an early gene accelerating expression of late genes of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol 1988;62:2773-81.
- Wu TY, Lin DG, Chen SL, Chen CY, Chao YC. Expression of highly controllable genes in insect cells using a modified tetracycline-regulated gene expression system. J Biotechnol 2000;80:75-83.
- Lee JC, Wu TY, Huang CF, Yang FM, Shih SR, Hsu JTA. High-efficiency protein expression mediated by enterovirus 71 internal ribosome entry site. Biotechnol Bioeng 2005;90:656-62.
- Jinn TR, Kao SS, Tzeng JTC, Wu TY. Coral red fluorescence protein as genetic modified baculovirus tracer. J Biotechnol 2005;119:255-9.
- Smith TJ, Piscatelli JJ, Andersen V, Wang HS, Lance PN. Butyrate induces plasminogen activator inhibitor type 1 messenger RNA in cultured Hep G2 cells. Hepatology 1996;23:866-71.
- Arts J, Lansink M, Grimbergen J, Toet KH, Kooistra T. Stimulation of tissue-type plasminogen-activator gene expression by sodium butyrate and trichostatina in human endothelial-cells involves histone acetylation. Biochem J 1995;310:171-6.
- Condreay JP, Witherspoon SM, Clay WC, Kost TA. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA 1999;96:127-32.
- Song SU, Boyce FM. Combination treatment for osteosarcoma with baculoviral vector mediate gene therapy (p53) and chemotherapy (Adriamycin). Exp Mol Med 2001;33:46-53.
- Hu YC. Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacol Sin 2005;26:405-16.
- Ho YC, Chen HC, Wang KC, Hu YC. Highly efficient baculovirus-mediated gene transfer into rat chondrocytes. Biotechnol Bioeng 2004;88:634-51.
- Ho YC, Chung YC, Hwang SM, Wang KC, Hu YC. Transgene expression and differentiation of baculovirus-transduced human mesenchymal stem cells. J Gene Med 2005;7:860-8.
- Dwayne DH, Tom AP, Jerrod H, David AT, Thomas AG. A series of broad host range shuttle vectors for constitutive and inducible expression of heterologous proteins in insect cell lines. Gene 1998;207:241-9.
- Iwanaga M, Takaya K, Katsuma S, Ote M, Tanaka S, Kamita SG, et al. Expression profiling of baculovirus genes in permissive and nonpermissive cell lines. Biochem Biophys Res Commun 2004;323:599-614.
