Icariin-mediated expression of cardiac genes and modulation of nitric oxide signaling pathway during differentiation of mouse embryonic stem cells into cardiomyocytes in vitro1
Introduction
Herba Epimedii is an important traditional Chinese herbal medicine that is widely used as a tonic, aphrodisiac, and antirheumatic in China. The content of icariin, its effective ingredient, has been used as a quality control standard. In our previous work, the specific properties of embryonic stem (ES) cells and standard culture methods[1] were used to confirm the inducible effects of icariin and its two metabolites on the differentiation of ES cells into cardiomyocytes in vitro[2]. Icariin at a concentration of 1×10-7 mol/L facilitates the directional differentiation of ES cells into cardiomyocytes. Cardiomyocytes are characterized by the expression of sarcomeric proteins, α-actinin and cardiac troponin T. Icariin has been found to have effects on regulation of the cell cycle, induction of apoptosis, as well as modulation of p53 during the early differentiation of ES cells and cardiac development, by using flow cytometry, reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analysis[3]. But the other possible mechanisms by which icariin inducibly affects the directional differentiation of ES cells into cardiomyocytes are still not completely understood and it is worthwhile to investigate them further.
Cardiac-specific genes, proteins, receptors and signaling molecules are expressed in a developmentally controlled manner during cardiac differentiation of ES cells in vitro, which closely recapitulate the developmental pattern of early cardiogenesis in vivo[4]. With a view to detecting the targets of icariin action during the promotion of ES cells to differentiate, the expression of cardiac developmental-dependent genes was measured using RT-PCR, and the chronotropic responses of cardiomyocytes to β-adrenoceptor stimulation were determined.
The second messengers, cAMP and cGMP, play an important part in regulating cellular proliferation and differen-tiation. It is crucial that the correct cAMP/cGMP ratio is maintained. Moreover, if a compound heightens the cAMP level of cells, it may facilitate differentiation and inhibit proliferation[5,6]. Therefore, the levels of cAMP and cGMP in ES cells treated with icariin were measured to clarify the possible actions of icariin on early differentiation events before a shift to the cardiomyocyte phenotype.
NO, a potential differentiation signal molecule, is important for cardiac development. It has been reported that incubation of embryoid body (EB) with nitric oxide synthase (NOS) inhibitors resulted in a pronounced differentiation arrest of cardiomyocytes, whereas this effect could be reversed by coapplication of the NO donor. In the development of cardiogenesis, the classic mode of action of NO is through intracellular signaling cascades via the activation of cGMP[7]. When administered orally, icariin can increase the mRNA and protein expression of eNOS and iNOS as a result of regulating the endogenous NO level to enhance erectile function[8]. In the present study, we tested the hypothesis that icariin promoted the differentiation of ES cells into cardiomyocytes in part through regulating the endogenous NO signaling pathway. The concentration of endogenous NO in ES cells during differentiation was evaluated by using the Griess reaction. In addition, aminoguanidine (AG), an effective and selective inhibitor of iNOS, was used to explore the influence of icariin on endogenous NO levels in the differentiation of ES cells into cardiomyocytes, and to confirm the targets of icariin action.
Materials and methods
Cells, drugs, and reagents The permanent ES cell line D3 (ES-D3) was obtained from the American Type Culture Collection (CRL-1934). Icariin was obtained from the Drug Biology Product Examination Bureau, Beijing, China (batch N
ES cell culture and icariin/aminoguanidine treatment ES-D3 cells were cultured in an undifferentiated state on primary cultures of mouse embryonic fibroblasts (MEF) cells in DMEM, supplemented with 10% FCS, 0.1 mmol/L β-mercaptoethanol, NEAA and 1×106 U/L LIF. Cultures of differentiating ES cells were established by the formation of EB in hanging drop cultures[2,9-11] with differentiation medium. On d 5, EBs were plated separately onto gelatin-coated 24-well culture plates, and at that time, icariin was added to the medium at concentrations of 1×10-7, 1×10-8, or 1×10-9 mol/L, on the basis of preliminary test results. EBs treated with 1×10-8 mol/L RA or with 0.1% dimethyl sulfoxide (Me2SO) solvent were used as positive or negative controls, respectively. To explore whether icariin promoted the differentiation of ES cells into cardiomyocytes in part through regulating the endogenous NO signaling pathway, AG at a concentration of 1×10-3 mol/L was added to differentiation medium together with icariin on d 5 and maintained until d 5+11 (ie, 11 d after EB was plated onto gelatin-coated culture plates on d 5). EB was observed with a light microscope every day to record the number of spontaneously beating EB.
In the experiment, d 1 corresponds to the day of dissociation of ES cells from MEF cells, and the initiation of differentiation by the formation of EB. To identify whether the observed cell types derived from ES-D3 cells were cardiomyocytes, indirect immunofluorescence for cardiac-specific protein was carried out according to a method published elsewhere[2,3].
Detection of cardiac-specific transcripts by semi-quantitative RT-PCR analysis Total RNA was isolated from EBs (n=20) induced by 1×10-7, 1×10-8 or 1×10-9 mol/L icariin, 1×10-8 mol/L RA or solvent at different time points by using Trizol reagent. After extraction, mRNA was precipitated and dissolved in a 0.1% diethylpyrocarbonate solution. To synthesize first strand cDNA, 7 µL total RNA was incubated in 0.5 µg of oligo (dT)6 primer and 5 μL deionized water at 65 °C for 15 min. Reverse transcription reactions were performed with 200 U M-MuLV reverse transcriptase in 5× reaction buffer and 1 mmol/L dNTP mixture for 1 h at 42 °C. Multiplex polymerase chain reaction mixtures of 50 µL contained 1 µL of the RT reaction product, 10×PCR buffer, 25 U Taq polymerase, 1 µL of 10 mmol/L dNTP mixture, and 30 pmol of each primer. The specific primer pairs designed by using Primer 3.0 software and published sequences were as follows (Table 1):
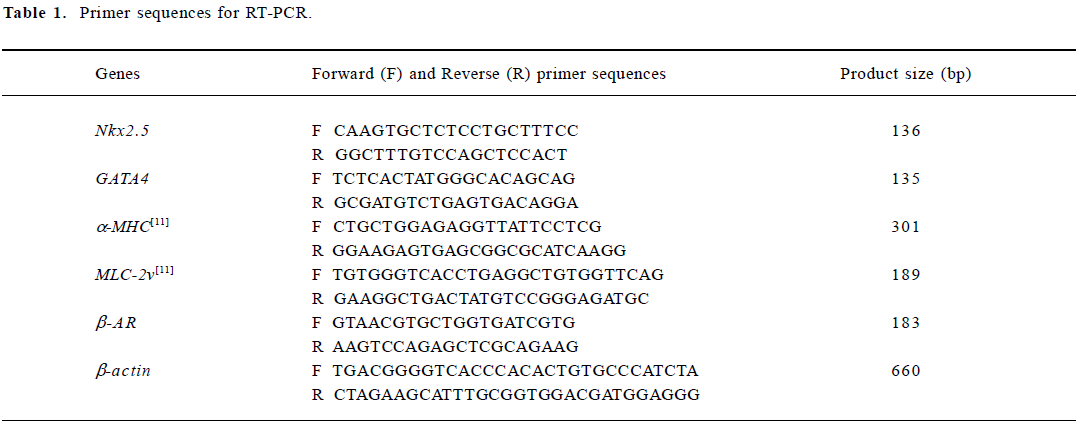
Full table
For the semi-quantitative determination of Nkx2.5, GATA4, α-MHC, MLC-2v and β-AR mRNA levels, the products of the reverse transcription reactions were denatured for 3 min at 94 °C, followed by 35 cycles (Nkx2.5), 35 cycles (GATA4), 40 cycles (α-MHC), 45 cycles (MLC-2v), 40 cycles (β-AR) or 30 cycles (β-actin) of amplification with Ampli Taq DNA polymerase as follows: 45 s denaturation at 94 °C, 40 s annealing at 54.0 °C (Nkx2.5 and GATA4), 66.4 °C (a-MHC), 61.1 °C (MLC-2v), 54.0 °C (β-AR) or 55.0 °C (β-actin), then 45 s elongation at 72 °C. The PCR products were separated by 1.5% agarose gel electrophoresis, visualized with ethidium bromide staining, and quantified using a Bio-imaging Analyzer (Bio-Rad,CA,USA), and the density of the products were quantitated using Quantity One (version 4.2.2) software (Bio-Rad).
Measurement of contraction of cardiomyocytes derived from ES-D3 cells The in vitro function of ES-D3 cell-derived cardiomyocytes was examined by evaluating the chronotropic effects of cardio-active drugs[12]. Isoprenaline (a β-AR agonist) was cumulatively added to the pulsating clusters of EB induced by 1×10-7 mol/L icariin, 1×10-8 mol/L RA or solvent on d 5+5, d 5+9, and d 5+13, respectively. Then the frequencies were measured in control (ie, the basal level of beating frequency, before agonist treatment) and agonist-treated variants to create dose–response curves. Measurements were also carried out in the presence of the antagonist propranolol. Images of the beating cardiomyocytes were monitored with a charge coupled device (CCD) video camera attached to the microscope observation port, and also displayed on a computer screen. The frequency of contraction was detected by using a photoelectricity transformation system, which transformed photic signals into electrical signals[13].
cAMP and cGMP determination assay To clarify the mechanism by which icariin affects the early differentiation of ES cells before a shift to the cardiomyocyte phenotype, cAMP and cGMP levels in ES cells were measured by using a 125I-cAMP and 125I-cGMP double antibody radioimmunoassay method[14]. ES-D3 cells were cultivated without MEF feeders and LIF, and treated with 1×10-7 mol/L icariin or 1×10-8 mol/L RA. After 24–48 h, the cells were harvested, placed into a polypropylene tube and centrifuged. The supernatant was removed and 1 mL acetic acid buffer (pH 4.5) at 4 °C was added to the cell pellet. Then samples were repeatedly frozen in liquid nitrogen and quickly melted in warm water (5 times). Protein was removed by centrifugation at 5000×g for 30 min.
Measurement of supernatant NO production Supernatant NO concentration was measured by using the Griess reaction[14]. The nitrite concentration was determined by using a curve calibrated with a sodium nitrite standard.
Statistical analysis Data were expressed as Mean±SD. At least 3 independent experiments were carried out to calculate mean. Statistical significance was evaluated with one-way ANOVA by using SPSS 10.0 for Windows. P<0.05 was considered statistically significant.
Results
Identification of cardiomyocytes derived from ES-D3 cells Apparent cell morphological changes during the course of differentiation were shown in Figure 1. The differentiated beating cardiac cells stained positively with anti-α-actinin mAb. These results accorded with those of our previous studies[2,3].
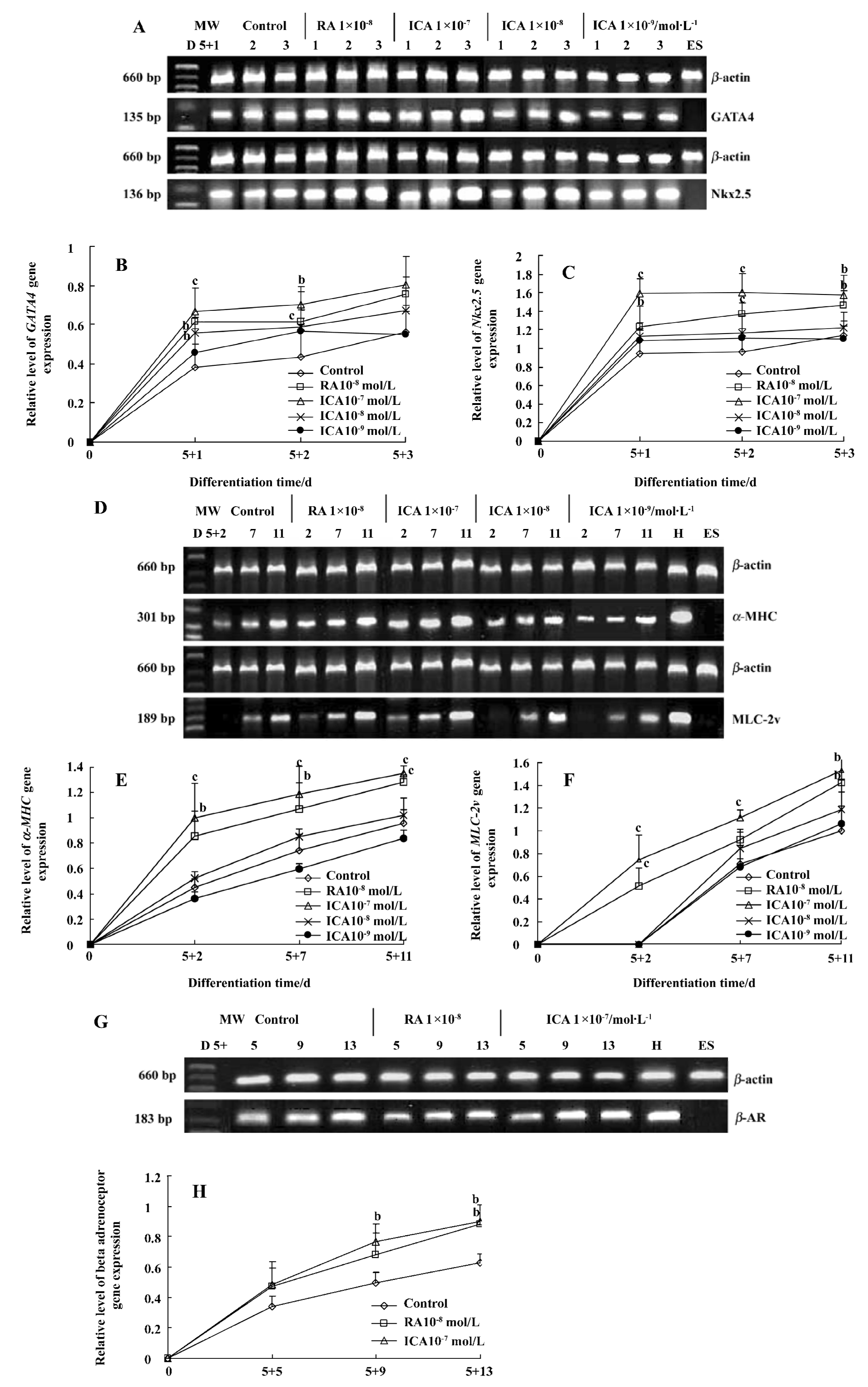
Expression of cardiac-specific genes during the early cardiac developmental stage Cardiomyocytes from EB expressed the cardiac transcription factors GATA4 and Nkx2.5, as well as the cardiac-specific genes α-MHC, MLC-2v and β-AR. Icariin significantly upregulated the mRNA levels of transcription factors GATA4 and Nkx2.5 from the onset of cardiac differentiation (P<0.01). The mRNA levels of α-MHC, MLC-2v and β-AR of cells in the presence of icariin increased during the early cardiac developmental stage in a concentration- and time-dependent manner (P<0.05). In particular, 1×10-7 mol/L icariin-induced MLC-2v expression was detected as early as d 5+2, whereas increased expression in control cells was not observed until d 5+7 (Figure 2).
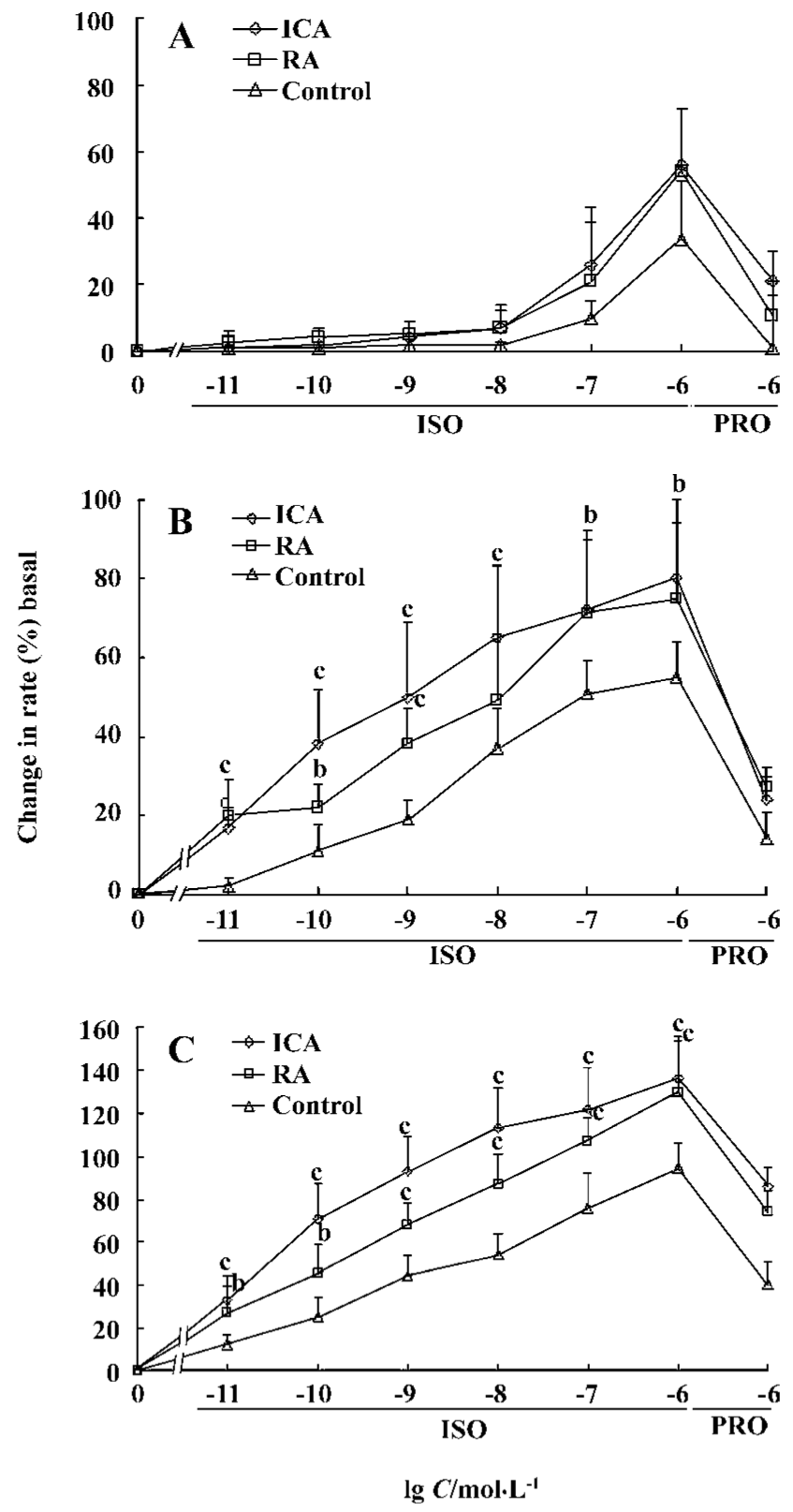
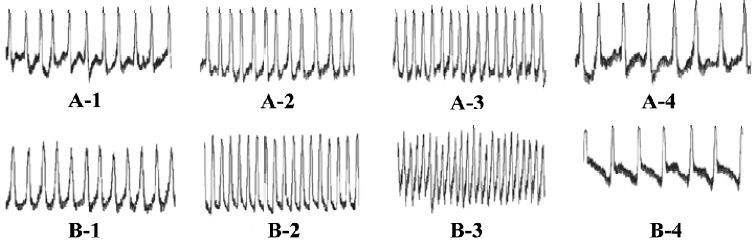
Chronotropic responses of ES-D3 cell-derived cardiomyocytes The spontaneously beating capacity of ES cell-derived cardiomyocytes enables the investigation of the chronotropic effect of cardioactive substances by measuring the beating frequency of cardiac cells[12]. A positive chronotropic response was observed after administration of the β-agonist isoprenaline. Furthermore, the effect of isoprenaline on ES-D3 cell derived cardiomyocytes was different in the early and late developmental stages. The beating EBs early on d 5+5 were insensitive to isoprenaline (Figure 3A). In an intermediate phase (d 5+9), the beating frequency was significantly increased in the presence of isoprenaline in a concentration-dependent manner (P<0.01). The frequency of beating EB treated with 1×10-7 mol/L icariin was increased by 20% when 1×10-11 mol/L isoprenaline was added. As the concentration of isoprenaline increased gradually to 1×10-6 mol/L, the frequency was increased by approximately 80%, whereas the corresponding increase was only 50% for controls (Figure 3B). At the terminal differentiation stage (d 5+13), the cardiomyocytes were highly sensitive to isoprenaline (P<0.01). In the presence of isoprenaline at concentrations ranging from 1×10-11 to 1×10-6 mol/L, the contraction rate of cardiomyocytes induced by 1×10-7 mol/L icariin increased from approximately 30% to 130% (Figure 3C). Propranolol (a β-adrenoceptor antagonist) at a concentration of 1×10-6 mol/L caused a reduction in the frequency increase. A representative trace showing the effect of isoprenaline and propranolol on the beat frequency of the cardiomyocytes is shown in Figure 4.
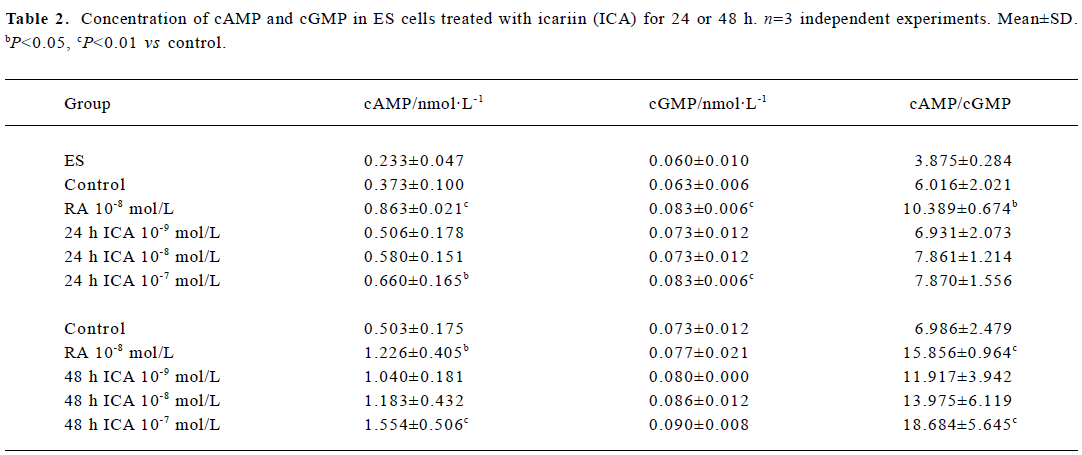
Levels of cAMP and cGMP in ES cells The concentrations of cAMP and cGMP in ES cells were 0.233 nmol/L and 0.060 nmol/L, respectively, and the cAMP/cGMP ratio was 3.875. When ES cells were treated with icariin at a concentration of 1×10-7 mol/L for 24 h, the levels of cAMP and cGMP significantly increased to 0.660 nmol/L and 0.083 nmol/L, respectively (P<0.05). The cAMP/cGMP ratio also increased to 7.870. Treatment of ES cells with icariin for 48 h caused elevation of the concentration of cAMP and the ratio of cAMP/cGMP (P<0.01), but the concentration of cGMP was barely increased (Table 2).
Full table
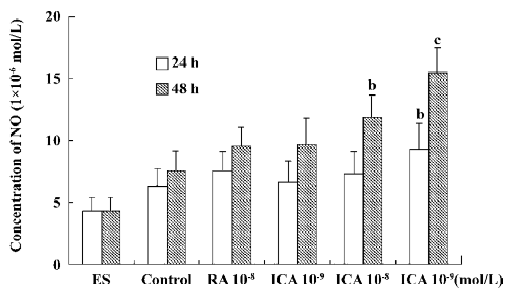
Production of supernatant NO In the control, supernatant NO production was low. When ES cells were treated with icariin at a concentration of 1×10-7 mol/L for 24 h or 48 h, supernatant NO production was markedly elevated (P<0.05). In particular, the NO-cGMP pathway was involved in the early differentiation of ES cells treated with icariin for 24 h. During the course of differentiation (from d 5+2 to d 5+11), icariin facilitated the directional differentiation of ES cells into cardiomyocytes, and supernatant NO generation increased (P<0.05). In the presence of AG, an effective and selective inhibitor of iNOS, NO generation during differentiation decreased significantly, but was rescued by icariin (P<0.05; Figures 5 and 6).
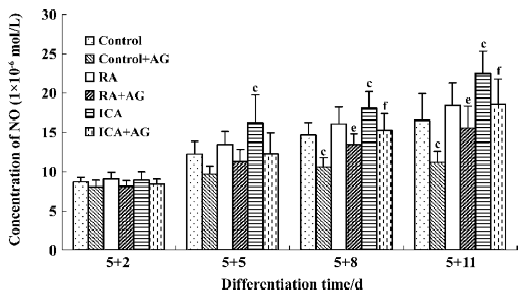
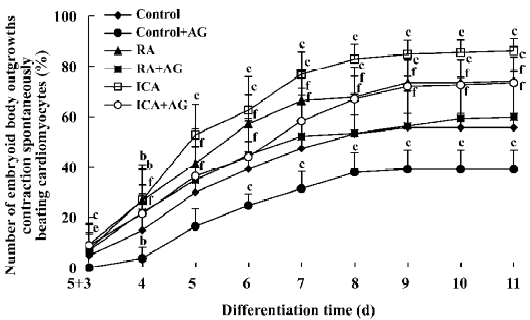
Inducible effect of icariin together with AG on the directional differentiation of ES-D3 cells into cardiomyocytes To further determine the effects of icariin on modulation of the endogenous NO signaling pathway during the differentiation of ES cells into cardiomyocytes, cultures were supplemented with AG to partially block NO production. Under these conditions, whether the percentage cardiac differentiation was influenced, and the number of spontaneously beating EB were recorded. ES cells were induced into rhythmically beating EB by icariin in a concentration- and time-dependent manner[3]. The percentage differentiation in cultures containing contracting EB with 1×10-7 mol/L icariin was approximately 7% on d 5+3, and reached a peak level of 86% on d 5+11 (P<0.05). In the control, only approximately 55% beating EBs were found on d 5+11. AG significantly delayed and decreased the incidence of contracting EB compared with control cells: beating EB was not observed until d 5+4 and the percentage was only approximately 35% on d 5+11. When icariin-treated cells were co-cultured with AG, AG produced a decrease in the percentage of beating EB to 73% on d 5+11 compared with cells treated with icariin alone. The potential of EB to undergo cardiac differentiation was significantly enhanced in comparison with that in solvent together with AG treatment starting from d 5+3 (P<0.05; Figure 7).
Discussion
Our experiments demonstrated that icariin markedly enhanced the mRNA levels of GATA4, Nkx2.5, α-MHC, MLC-2v and β-AR at the early stage of differentiation. It has been reported that the GATA4 and Nkx2.5 transcription factors appear before other cardiac genes are expressed[15]. Myocardial genes such as α-MHC are expressed relatively early during the course of cardiogenesis[16]; however, MLC-2v mRNA is expressed at high levels relatively late, and is spatially restricted to the ventricular area[17]. In the present study RT-PCR analysis revealed that cardiac differentiation of ES cells induced by icariin recapitulated this developmental pattern, as GATA4 and Nkx2.5 were detected earlier than cardiac α-MHC, MLC-2v and β-AR. Icariin-induced MLC-2v expression upregulation was detected as early as d 5+2, whereas increased expression in control cells was not observed until d 5+7. These results suggest that one of the mechanisms by which icariin is involved in differentiation is by facilitating the expression of the cardiac developmental-dependent genes in a developmentally controlled manner. In particular, icariin could enhance the development of ventricular cardiomyocytes at an early differentiation stage.
It is important to characterize the responses of cardiomyocytes derived from ES cells, and one of the key modulators of contraction is the β-AR system[18,19], therefore in the present study the responses of the beating rate of cardiomyocytes to β-AR stimulation were determined. We confirmed that β-AR responses were well developed in cardiomyocytes derived from ES cells. The cardiomyocytes treated with icariin were more sensitive to isoprenaline than the case of in controls. The different sensitivities of the cardiomyocytes to isoprenaline may result from the different expression levels and the degree of maturity of β-AR[20], the hypothesis that is supported by the results of our RT-PCR analysis.
NO is a potential differentiation signal that plays an important role in the differentiation of ES cells into cardiomyocytes[21]. In most instances, NO signaling pathways are typically dependent on cGMP. The cGMP-dependent pathways are initiated by NO-induced activation of soluble guanylate cyclase by binding to heme iron. This activation results in a transient increase in the second messenger cGMP[22]. Another messenger, cAMP, also plays an important role in regulating cellular proliferation and differentiation. The effects of cAMP and cGMP are opposite. It is crucial to maintain the correct cAMP/cGMP ratio to provide the correct microenvironment for cell survival and function. Upregulation of cAMP is a key process for facilitating differentiation and inhibiting proliferation. Icariin induces the differentiation of HL-60 cells, possibly by elevating the cAMP/cGMP ratio[5]. In the present study, treatment of ES cells with icariin for 24–48 h resulted in an elevated cAMP concentration and cAMP/cGMP ratio. This indicates that upregulation of cAMP concentration and cAMP/cGMP ratio is important for the differentiation of ES cells in early differentiation events, before a shift to the cardiomyocyte phenotype. In the present study, when ES cells were treated with icariin for 24–48 h before a shift to the cardiomyocyte phenotype, supernatant NO production was markedly elevated. The cGMP level was also elevated with icariin treatment for only 24 h. On the basis of the results of the radioimmunoassays and the Griess reaction measurements, we suggest that the NO-cGMP pathway is only involved in the early differentiation of ES cells treated with icariin for 24 h before a shift to the cardiomyocyte phenotype. However, the fact that the involvement of NO was independent of cGMP in early differentiation warrants further investigation.
Further studies were aimed at the involvement of icariin in the NO signaling pathway during cardiomyocyte phenotype formation. NO is generated by the NOS family of proteins. Both NO and NOS isoforms have been shown to induce or facilitate the differentiation of several cell types, including nerve cells[23], some tumor cell types[24], and cardiac cells. ES cell-derived cardiomyocytes have been found to have an identical pattern of NOS expression as that detected in mouse heart. In the early developmental stage, ES cell-derived cardiomyocytes displayed strong iNOS and eNOS expression. Conversely, in the late developmental stage, iNOS was not detected and eNOS expression was found only at low levels. iNOS was expressed only in the early cardiac differentiation stage and was important for cardiac development. Work with mouse ES cells has shown that NOS inhibitors arrest differentiation toward the cardiac phenotype, and this effect can be rescued by NO donors[21]. Orally administered icariin has been shown to increase the mRNA and protein expression of eNOS and iNOS and regulate the level of NO to enhance erectile function[8]. However, whether icariin improves cardiovascular function in part through regulating the level of NO has not been demon-strated. In the present study, an in vitro model was used to investigate the influence of NOS inhibitors on cardiac development within EB treated with icariin. AG is an effective and specific inhibitor of iNOS. During the course of differentia-tion, icariin facilitated the directional differentiation of ES cells into cardiomyocytes and supernatant NO concentration increased. AG decreased the endogenous NO level and arrested differentiation toward the cardiac phenotype. This effect of AG could be rescued by icariin. These findings suggest that icariin promotes the differentiation of ES cells into cardiomyocytes in part through upregulating the endogenous NO level, which may be related to inducing iNOS expression. They also indicate that NO plays an important role in normal cardiac development as a potential differentiation signaling molecule.
In conclusion, the promoting effects of icariin on cardiac differentiation depended on advancing and increasing the expression of the GATA4, Nkx2.5, α-MHC, MLC-2v and β-AR genes. Icariin could also increase cAMP concentration and the cAMP/cGMP ratio, and promote endogenous NO generation during early differentiation and cardiac development.
References
- Evans MJ, Kaufman MH. Establishment in culture of pluripo-tential cells from mouse embryos. Nature 1981;292:154-6.
- Zhu DY, Lou YJ. Inducible effects of icariin, icaritin, and demethylicaritin on directional differentiation of embryonic stem cells into cardiomyocytes in vitro. Acta Pharmacol Sin 2005;26:477-85.
- Zhu DY, Qu LH, Zhang XN, Lou YJ. Icariin-mediated modulation of cell cycle and p53 during cardiomyocyte differentiation in embryonic stem cells. Eur J Pharmacol 2005;514:99-110.
- Hescheler J, Fleischmann BK, Lentini S, Maltsev VA, Rohwedel J, Wobus AM, et al. Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovasc Res 1997;136:149-62.
- Zhao Y, Cui Z, Zhang L. Effects of icariin on the differentiation of HL-60 cells. Natl Med J Chin 1997;19:53-5. Chinese.
- Lopez-Lluch D, Fernandez-Ayala DJM, Alcain FJ, Buron MI, Quesada JM, Navas P. Inhibition of COX activity by NSAIDs or ascorbate increases cAMP levels and enhances differentiation in 1a, 25-dihydroxyvitamin D3-induced HL-60 cells. Arch Biochem Biophys 2005;436:32-9.
- Bloch W, Fleischmann BK, Lorke DE, Andressen C, Hops B, Hescheler J, et al. Nitric oxide synthase expression and role during cardiomyogenesis. Cardiovasc Res 1999;43:675-84.
- Tian L, Xin ZC, Liu WJ, Yang YM, Liu G, Chen L, et al. Effects of icariin on the erectile function and expression of nitrogen oxide synthase isoforms in corpus cavernosum of arterigenic erectile dysfunction rat model. Zhonghua Yi Xue Za Zhi 2004;84:954-7. Chinese..
- Metzger JM, Lin WI, Johnston RA, Westfall MV, Samuelson LC. Myosin heavy chain expression in contracting myocytes isolated during embryonic stem cell cardiogenesis. Circ Res 1995;76:710-9.
- Scholz G, Pohl I, Genschow E, Klemm M, Spielmann H. Embryotoxicity screening using embryonic cells in vitro: correlation to in vivo teratogenicity. Cells Tissues Organs 1999;165:203-11.
- Wobus AM, Kaomei G, Shan J, Wellner M, Rohwedel J, Guanju J, et al. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol 1997;29:1525-39.
- Kaomei G, Jurgen R, Anna MW. Embryonic stem cell differentiation models: cardiogenesis, myogenesis, neurogenesis, epithelial and vascular smooth muscle cell differentiation in vitro. Cytotechnology 1999;30:211-26.
- Jing XK, Alexander L. Protective effects of all-trans-retinoic acid against cardiac arrhythmias induced by isoproterenol, lysophosphatidylcholine or ischemia and reperfusion. J Cardiovasc Pharmacol 1995;26:943-8.
- Chen Y, Wang HL, Lou YJ. Signal transduction pathways induced by nitric oxide in rat hepatocytes. J Zhejiang Univ 2002;31:424-8. (Med Sci).
- Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 2002;91:189-201.
- Lyons GE, Schiaffino S, Sassoon D, Barton P, Buckingham M. Developmental regulation of myosin gene expression in mouse cardiac muscle. J Cell Biol 1990;111:2427-36.
- O’Bruen TX, Kee K, Chien KR. A morphogenetic field of MLC-2v gene expression in the primordial heart tube of normal and MLC-luciferase transgenic mice. Proc Natl Acad Sci USA 1993;90:5157-61.
- Ali NN, Xu X, Brito-Martins M, Poole-Wilson PA, Harding SE, Fuller SJ. Beta-adrenoceptor subtype dependence of chronotropy in mouse embryonic stem cell-derived cardiomyocytes. Basic Res Cardiol 2004;99:1-10.
- Wobus AM, Wallukat G, Hescheler J. Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation 1991;48:173-82.
- Maltsev VA, Ji GJ, Wobus AM, Fleischmann BK, Hescheler J. Establishment of b-adrenergic modulation of L-type Ca2+ current in the early stages of cardiomyocyte development. Circ Res 1999;84:136-45.
- Kanno S, Kim PKM, Sallam K, Lei J, Billiar TR, Shears LL. Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc Natl Acad Sci USA 2004;101:12277-81.
- Murad F. Cellular signaling with nitric oxide and cyclic GMP. Braz J Med Biol Res 1999;32:1317-27.
- Peunova N, Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature 1995;375:68-73.
- Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood 1992;80:1880-4.
