Antitumor effect of cytosine deaminase/5-fluorocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma1
Introduction
Suicide gene therapy is a highlight in tumor gene therapy at present. Therapy using cytosine deaminase/5-fluorocyto-sine (CD/5-FC) is one of the most widely studied systems, in which CD can convert relatively nontoxic 5-fluorocytosine (5-FC) into a toxic metabolite 5-fluorouracil(5-FU) and kill tumor cells[1–4].
Transfer vectors are a key factor in the tumor gene therapy system, which directly influences tumor targeting. It is evident that most of the interest and effort in cancer gene therapy in the coming years should be focused on transfer vectors. So far viruses are the most widely used suicide gene vectors. However, viruses are relatively poor in tumor targeting and safety, which has badly impeded tumor gene therapy[5,6]. It is crucial to search for a novel gene vector that has good tumor targeting and is relatively safe.
It is evident that the center of a solid tumor is generally found at a low level of oxygen, and anaerobic bacteria tend to colonize in a low oxygen environment. Therefore, anaerobic bacteria is a potential vector for tumor gene therapy[7–9]. In a previous study, our laboratory selected a strain of Bifidobacterium Infantis proven to be good for targeting solid tumors and non-pathogenic[10]. The aim of the present study was to transfer the CD gene into Bifidobacterium infantis to construct a Bifidobacterium infantis/CD targeting gene therapy system and observe the antitumor efficacy of a CD/5-FC suicide gene therapy system mediated by Bifidobac-terium infantis on melanoma in vitro and in vivo.
Materials and methods
Materials pGEX-1LamdaT plasmid was kindly provided by Research Unit of Infection & Immunity, West China School of Preclinical and Forensic Medicine, Sichuan University, China. Strains of E Coli K12λ were purchased from Chengdu Institute of Biological Products, China. Strains of Bifidobacterium Infantis 2001 were obtained from West China School of Stomatology, Sichuan University, China.
LA Taq DNA polymerase, T4 DNA ligase and λ-EcoT14 I digest DNA Marker were purchased from TaKaRa, Japan. 200 bp DNA Ladder Marker was purchased from Sino-American Biotechnology, China. Purification kit of plasmid, Wizard PCR Preps DNA Purification Resin, EcoR I and BamH I were purchased from Promega (Madison, WI, USA).
Mouse melanomacytes B16-F10 were provided by China Center for Typical Culture Collection. Female C57BL/6 mice (class 1, aged 6–8 weeks) were provided by the Experimental Animal Center of Sichuan University.
Methods
Amplification of CD gene Strains of E Coli K12λ were inoculated into 5 mL LB liquid medium with shaking, overnight at 37 ºC. The next day, genomic DNA was prepared by phenol/chloroform method and used as template DNA to perform PCR for the amplification of CD gene. Specific primers of CD gene were designed based on published sequences (Genebank, NC-0009131). The upstream primer is 5'-ATG GAT CCG GAG GCT AAC AAT G-3' and the downstream primer is 5'-GGG GAA TTC TGT AAC CCA GTC GT-3'. The amplification cycle was repeated 35 times with the condition of denaturation at 94 ºC for 30 s, annealing at 55 ºC for 30 s and extension at 72 ºC for 2 min. Amplified products were purified with Wizard PCR Preps DNA Purification Resin and separated with electrophoresis of 0.8% agarose gel to confirm whether amplified products had the desired size of 1.3 kb.
Digestion of CD gene and pGEX-1LamdaT plasmid CD gene 1 µg and pGEX-1 LamdaT plasmid 1 µg were added into 10 µL 10×Buffer E reactions, separately. The plasmid and CD gene were digested with dual restriction endonucleases (1 µL EcoR I+1 µL BamH I) for 3 h. Once the digestion was complete, the DNA fragments were separated by electrophoresis with 0.8% agarose gel. The desired bands of 4.9 kb and 1.3 kb were cut out from the gel. The DNA fragments in the gel were extracted and recovered with gel extraction kit. Recovered pGEX-1LamdaT plasmid vector fragment was dephosphorylated with calf intestinal phosphatase.
Ligation of plasmid vector fragment and CD gene fragment Recovered CD gene fragment 10 µL, recovered pGEX-1LamdaT plasmid vector fragment 10 µL, and 1U T4 DNA ligase were added into the microfuge tube. The reactions were incubated at 16 ºC overnight. Then the ligation products, recombinant CD/pGEX-1LamdaT plasmid were separated by electrophoresis to confirm whether the ligation products had the desired size.
Transfection of Bifidobacterium infantis Suspensions of Bifidobacterium infantis 10 mL were washed with ice-cold pure water and resuspended in 40 µL ice-cold 10% glycerol. Recombinant CD/pGEX-1LamdaT plasmid 5 µL was added to the bacterial suspensions, then mixed and transferred to electroporation cuvette. Electroporation was carried out to transfect recombinant CD/pGEX-1LamdaT into Bifidobacterium infantis at 2.0 kV for 10 ms.
Culture of transfected Bifidobacterium infantis and detection of positively transfected bacterial colony When electroporation was completed, 1 mL MRS liquid medium was added into the electroporation cuvette to resuspend bacteria. Then the bacteria suspensions were transferred into a 1.5 mL EP tube and incubated in an anaerobic jar at 37 ºC. Two hours later, bacteria suspensions were plated on MRS solid medium with ampicillin and incubated in an anaerobic jar at 37 ºC. After 72 h, a single positively transfected bacterial colony of Bifidobacterium infantis was picked up and inoculated into 5 mL MRS liquid medium. Two to three drops of liquid paraffin was added to the medium and then the cultures were incubated overnight at 37 ºC. The next day, recombinant plasmid was extracted from the incubated bacteria using Purification kit of plasmid. Extracted recombinant plasmid was separated with electrophoresis to confirm whether the recombinant plasmid is the recombinant CD/pGEX-1LamdaT plasmid. In addition, the extracted recombinant plasmid from positively transfected bacterial colony was digested with dual restriction endonucleases of EcoR I and BamH I as described previously. The size of digested segments were analyzed by electrophoresis with 0.8% agarose gel.
Sequencing of inserted gene segment in recombinant plasmid Sequencing of inserted gene segment in recombinant plasmid extracted from positively transfected bacteria was performed according to the method of Sanger dideoxy-nucleotide triphosphate chain termination. Shanghai Genebase Gen-tech (Shanghai, China) carried out the sequencing.
Processing of positively transfected Bifidobacterium infantis Suspension of positively transfected Bifidobac-terium infantis 20 mL was incubated at 37 ºC anaerobically overnight. The next day, 5-FC with final concentration 1 mmol/L was added and then the incubation continued. After 24 h, 10 mL bacteria suspension was taken and centrifuged for 5 min. The supernatant fluid was collected and 2 mL ethyl acetate was added and mixed to make the supernatant fluid suspension in ethyl acetate properly. The mixture was centrifuged (150×g) at 4 ºC for 10 min. The organic fluid was transferred to EP pipette and dried in a vacuum. The dried product was resolved in 1 mL 0.9% saline to be used as the positive treatment fluid. Suspension of untransfected Bifidobacterium infantis was processed with the same method and used as the negative treatment fluid.
Killing effects of treatment fluid on melanomacyte B16-F10 Melanomacytes B16-F10 were first digested with 0.125% trypsin and counted. They were then diluted to a final concentration of 1.5×105 cells/mL and inoculated into six wells at 1.8 mL/well in 6-well cell culture cluster. The six wells were randomized into two groups with three wells in each group. When 50%–60% of the wells were covered by cells, 200 µL positive treatment fluid and 200 µL negative treatment fluid were separately added to the two groups with one well from each group added with saline as the control. The cell culture cluster was placed in a 5% CO2 incubator at 37 ºC. After 24 h, the morphology of cells was observed and the number of cells was counted using trypan blue dye exclusion method to calculate the suppression rate of each test group, namely; Suppression rate=(Nn–N)/(Nn–N0)×100% (N0: number of inoculated cells; N: number of cells in test well after 24 h; Nn: number of cells in control well after 24 h).
Antitumor effect in vivo Mouse melanomacytes B16-F10 were inoculated in the muscle of the right thigh of the female C57BL/6 mice at 5×106 cells/mouse to establish a melanoma model. The experiment was initiated when the tumor grew to 0.8–1.0 cm in diameter (d 10 after inoculation). Twenty tumor-bearing mice were divided into test group(n=10) and control group (n=10) randomly. Diluted suspension 0.2 mL of positively transfected Bifidobacterium infantis containing 5×106–6×106 bacteria was injected into the mice in the test group through the tail vein. The control group were injected with the same volume and number of untrans-fected Bifidobacterium infantis. After 7 d, 5-FC was injected intraperitoneally for each mouse in both groups at a dose of 500 mg each day, consecutively for 7 d. From the first day of 5-FC injection, the length and width of the tumor were measured every two days. The volume was calculated using the formula V=1/2×A×B2 (A: length; B: width) and the measurement continued for 21 d.
Statistic methods Comparison of tumor growth suppression rates was examined by chi-square test of four-fold table. Tumor volume was compared by t-test. The results were analyzed using statistic software STATA5.0.
Results
Detection of positively transfected Bifidobacterium infantis colony Electroporation method was used to transfect recombinant CD/pGEX-1LamdaT plasmid into Bifidobac-terium infantis. After 72 h, sparse bacterial colonies were observed on MRS solid medium containing ampicillin. A single positively transfected bacterial colony was picked up and cultured, then the recombinant plasmid in the positively transfected Bifidobacterium infantis was extracted. The size of extracted recombinant plasmid was approximately 6.2 kb (Figure 1, lane A), which was equal to the size of recombinant CD/pGEX-1LamdaT plasmid.
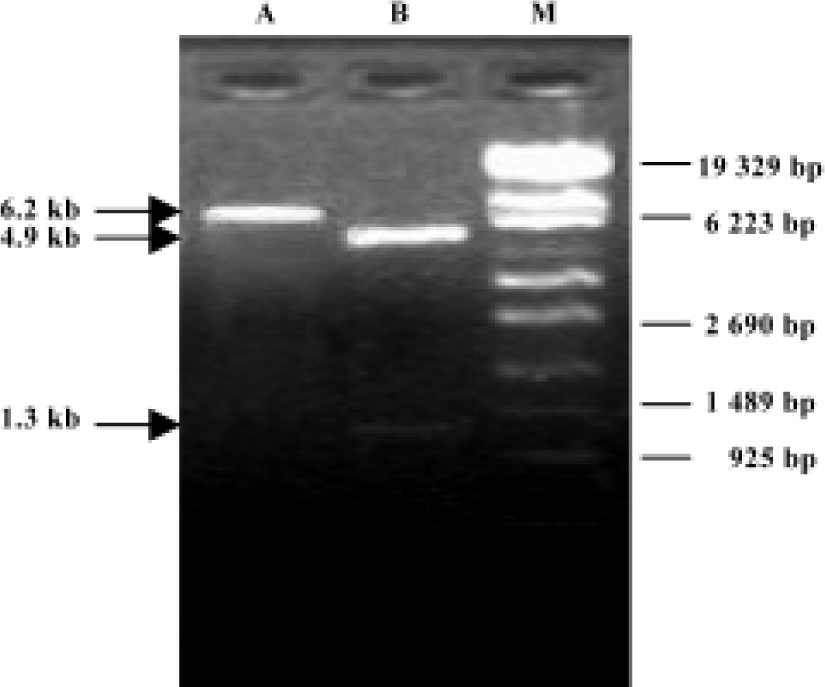
Detection of recombinant plasmid digested with dual restriction endonucleases After the recombinant plasmid extracted from the positively transfected bacteria was digested with dual restriction endonucleases of BamH I and EcoR I, two fragments with sizes of approximately 1.3 kb and 4.9 kb were obtained, which were consistent with the sizes of CD gene and pGEX-1LamdaT plasmid, respectively (Figure 1, lane B).
Sequencing of inserted gene segment in recombinant plasmid extracted from positively transfected Bifidobac-terium infantis Sequencing results showed that the size and sequence of nucleotide acid of inserted gene segment were completely consistent with the size and sequence of nucleotide acid of CD gene (Genebank: NC-000913). The full length of inserted gene fragment was 1309 bp. The sequence of two ends of the inserted gene was also consistent with BamH I site and EcoR I site. The sequencing identified that the foreign gene, CD gene, was correctly inserted into pGEX-1LambdaT plasmid and transferred into Bifidobacterium Infantis. Targeting gene therapy system of Bifidobacterium Infantis/CD was successfully constructed.
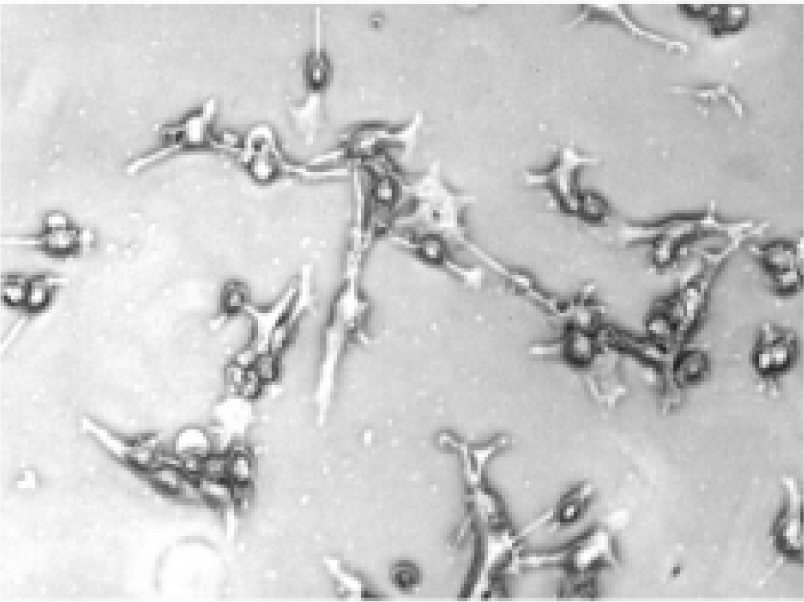
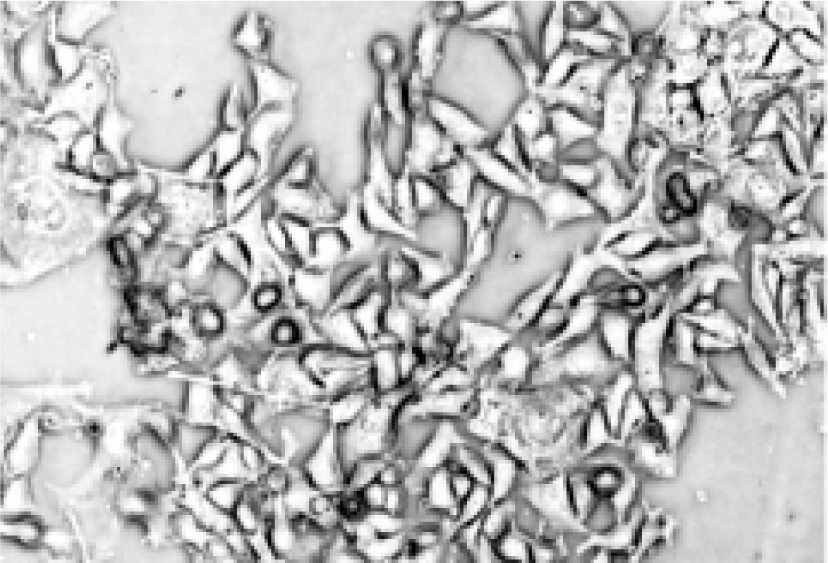
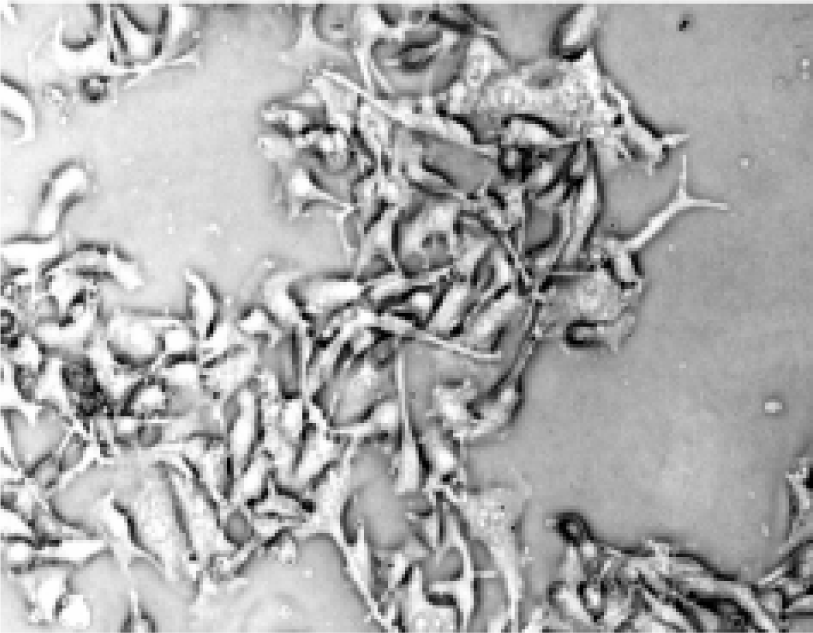
Morphologic changes of B16-F10 cells after treatment After being treated with the positive treatment fluid for 24 h, most B16-F10 cells were killed and only a few grew along the wall. Most cells floated and quantities of cell debris were observed in the medium. The cells left on the wall underwent significant changes in morphology; the original shape was gone, cytoplasm became rougher, the nucleus showed pycnosis and the refraction decreased (Figure 2), demonstrating obvious cellular damages. In contrast, B16-F10 cells treated with negative treatment fluid did not appear to have obvious morphologic changes compared with the control (Figures 3, 4).
Examination of tumor cell growth suppression rate The negative treatment fluid had no significant effects on tumor cell growth. The cells growth suppression rate was 4.8%. The B16-F17 cells treated with positive treatment fluid grew at a critically lower rate. The cells growth suppression rate was 80%, which was significantly higher than that of group treated with negative treatment fluid (chi-square test: χ2=7.02, υ=1, P<0.01) (Table 1).
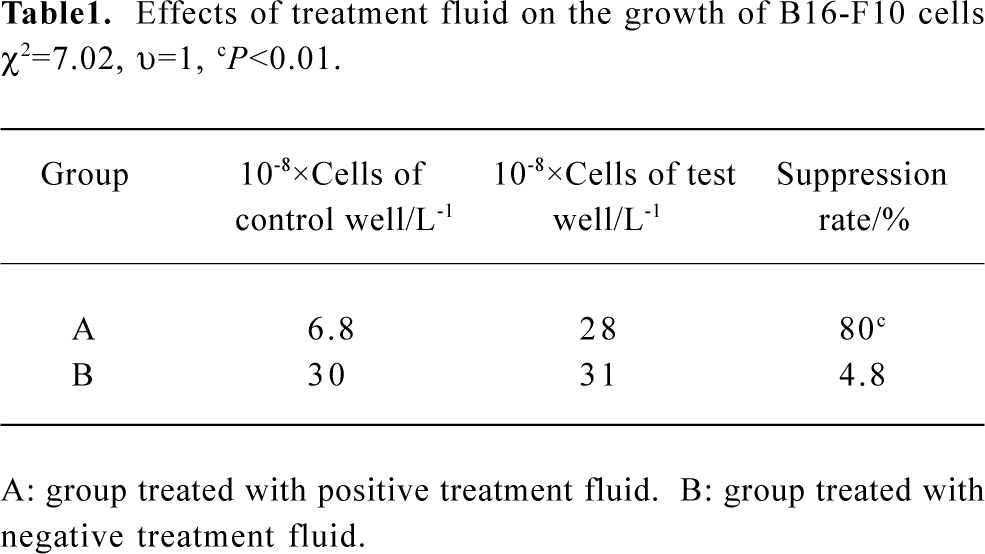
Full table
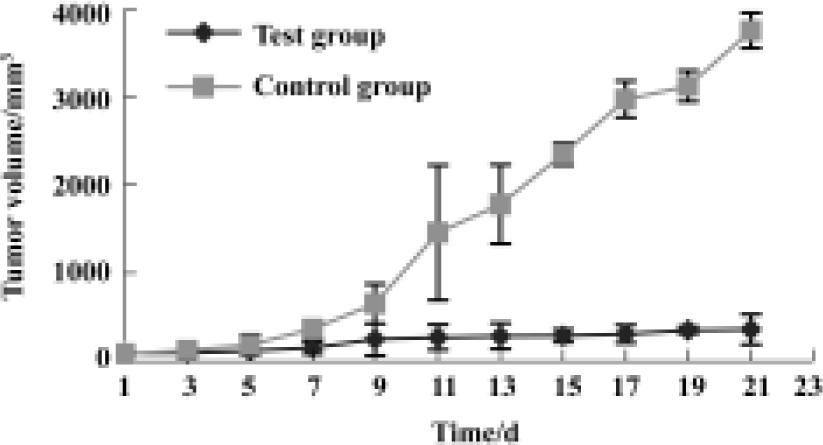
Antitumor effects in vivo There was no significant difference between the tumor volumes in the test group and control group before treatment. On the d 7 after 5-FC injection, tumor volumes in the test group were considerably smaller than those in the control group (P<0.05), and the difference became greater over time until the end of observation (Figure 5).
Discussion
It has been proved that a hypoxic region exists in many human and mouse tumors, especially in solid tumors, which kill over 90% of cancer patients. Vaupel[11] made his study on cancer patients using oxygen electrode measurement. In his study, Vaupel found the average oxygen partial pressure in normal tissues read 24–66 mmHg, whereas the readings dropping to 10–30 mmHg in a tumor tissue with a marked central region where the readings went below 2.5 mmHg. Based on the presence of a hypoxic metabolic region in a solid tumor and tendency of anaerobic bacteria to hypoxic environment, anaerobic bacteria can be used as transfer vectors for tumor targeting gene therapy.
Compared with viral or other non-viral vectors, the anaerobic bateria is advantageous for tumor targeting[13–17]. Yazawa et al[9] injected non-pathological strains of Bifidobacterium 105-A and 108-A into mice bearing lung cancer. After 168 h, both strains of bacteria were observed to have targeted and colonized in the tumors, whereas no bacteria were found in the liver, spleen, kidney, normal lung tissues or other normal tissues. When the anaerobic bacteria were used as the gene transfer vector, it could specifically proliferate and directly express foreign gene products in tumor tissues. There is no need to transform cancer cells, which highly improves the gene expression[9,13,14] . Most of the transfer vectors used in current studies are strains of Bifidobacterium. Bifidobac-terium is a non-pathogenic anaerobic bacteria. It resides in the lower section of the small intestine and in the large intestine of humans or rodent animals, and is good for the health of its host and widely used in food manufacturing and medicine[9,12–15]. It has been proved in animal experiments that Bifidobacterium has no obvious influence on body weight, peripheral leukocytes, temperature, or survival time of mice, hamsters, guinea pigs or rabbits[13]. Moreover, anaerobic bacteria are highly vulnerable to antibiotics and very low doses of antibiotics are enough to kill them. Even in the case of overgrowth, the bacteria can be easily controlled by antibiotics, which further the safety.
In the present study, we used Bifidobacterium infantis as a CD gene transfer vector, then transferred the CD gene into Bifidobacterium infantis and successfully constructed a Bifidobacterium infantis/CD targeting gene therapy system.
In the in vitro experiment, positively transfected Bifido-bacterium infantis was incubated anaerobically with 5-FC overnight. The supernatant fluid of the bacteria culture was extracted and resolved in saline. The final solution was added to the melanomacyte B16-F10 and it was observed that the growth of melanomacyte B16-F10 was obviously suppressed at a rate of 80% and the morphology of the cells was also damaged. It was also observed in our study that the growth of tumors were greatly inhibited on mice that were injected with 5-FC and positively transfected Bifidobacterium infantis. These results indicate that positively transfected with Bifidobacterium infantis can express CD in vitro and in vivo, which can convert 5-FC into 5-FU to inhibit tumor growth.
CD/5-FC suicide gene therapy system mediated by Bifidobacterium infantis has primarily demonstrated its antitumor effects in vivo and in vitro in this experiment. This system shows promise as a novel tumor targeting gene therapy system and is worthy of further study.
References
- Miller CR, Gustin AN, Buchsbaum DJ, Vickers SM, Manne U, Grizzle WE, et al. Quantitation of cytosine deaminase mRNA by real-time reverse transcription polymerase chain reaction: a sensitive method for assessing 5-fluorocytosine toxicity in vitro. Anal Biochem 2002;30:189-99.
- Miyagi T, Koshida K, Hori O, Konaka H, Katoh H, Kitagawa Y, et al. Gene therapy for prostate cancer using the cytosine deaminase/uracil phosphoribosyltransferase suicide system. J Gene Med 2003;5:30-7.
- Plumb JA, Bilsland A, Kakani R, Zhao J, Glasspool RM, Knox RJ, et al. Telomerase-specific suicide gene therapy vectors expressing bacterial nitroreductase sensitize human cancer cells to the pro-drug CB1954. Oncogene 2001;20:7797-803.
- Brown NL, Lemoine NR. Clinical trials with GDEPT: cytosine deaminase and 5-fluorocytosine. Methods Mol Med 2004;90:451-7.
- Xu G, McLeod HL. Strategies for enzyme/prodrug cancer therapy. Clin Cancer Res 2001;7:3314-24.
- Denny WA. Prodrug strategies in cancer therapy. Eur J Med Chem 2001;36:577-95.
- Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther 2002;9:291-6.
- Nuyts S, Van Mellaert L, Theys J, Landuyt W, Lambin P, Anne J. Clostridium spores for tumor-specific drug delivery. Anticancer Drugs 2002;13:115-25.
- Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S. Bifido-bacterium longum as a delivery system for cancer gene therapy: selective localization and growth in hypoxic tumors. Cancer Gene Ther 2000;7:269-74.
- Wu Y, Yi C, Wang S, Zhang M, Zhang J. Investigation on tumor-targeting characteristics of Bifidobacterium infantis toward melanoma in mice. J Sichuan Univ 2003;34:435-8.
- Vaupel PW. Oxygenation of solid tumors in drug resistance in oncology. In: Teicher BA, editor. New York: Marcel Dekker; 1993, p53–85.
- Nagy H, Panis Y, Fabre M, Perrin H, Klatzmann D, Houssin D. Are hepatomas a good target for suicide gene therapy? An experimental study in rats using retroviral-mediated transfer of thymidine kinase gene. Surgery 1998;123:19-24.
- Fujimori M, Amano J, Taniguchi S. The genus Bifidobacterium for cancer gene therapy. Curr Opin Drug Discov Devel 2002;5:200-3.
- Yazawa K, Fujimori M, Nakamura T, Sasaki T, Amano J, Kano Y, et al. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat 2001;66:165-70.
- Nakamura T, Sasaki T, Fujimori M, Yazawa K, Kano Y, Amano J, et al. Cloned cytosine deaminase gene expression of Bifido-bacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci Biotechnol Biochem 2002;66:2362-6.
- Kim TB, Song SH, Kang SC, Oh DK. Quantitative comparison of lactose and glucose utilization in Bifidobacterium longum cultures. Biotechnol Prog 2003;19:672-5.
- Makelainen H, Tahvonen R, Salminen S, Ouwehand AC. In vivo safety assessment of two Bifidobacterium longum strains. Microbiol Immunol 2003;47:911-4.