Inhibitory effect of agmatine on proliferation of tumor cells by modulation of polyamine metabolism1
Introduction
Polyamines, including putrescine, spermidine, and spermine, are required for cell proliferation and homeostasis. The intracellular pool of polyamines is precisely regulated through their biosynthesis, degradation, uptake, and excretion[1]. The disorder of intracellular polyamines plays an important role in carcinogenesis. Polyamines can promote the neoplastic transformation of normal cells, stimulate the proliferation of tumor cells, and facilitate angiogenesis in tumor tissues. Therefore, their metabolism pathway is an interesting anticancer drug target[2].
Agmatine, one of the analogs of polyamines, is the product of L-arginine decarboxylation and was initially believed to be present only in bacteria, plants, and invertebrates. Now it has been shown to be present in mammals[3]. The accumulated results show that agmatine has some important biological activities[4]. Among them, the inhibitory effect of agmatine on cell proliferation is of great interest.
The current results show that agmatine is able to modulate the cellular concentration of polyamines[5]. Agmatine can be hydrolyzed to putrescine and urea. Putrescine is then converted into spermidine and spermine by spermidine/spermine synthases[6]. So agmatine might have the capacity to increase the level of intracellular polyamines. In addition, agmatine has been postulated to decrease the cellular level of polyamines. There is much evidence to support this hypothesis. First, because agmatine and polyamines are structurally analogous and derived from same precursor, L-arginine[7], administration of exogenous agmatine would be able to reduce the synthesis of polyamines by a back-feed way. Second, as a competitor, agmatine can retard putrescine intake by the same carrier[8]. Most importantly, besides polyamines, agmatine is the only known molecule that has the capacity to induce antizyme[9]. Antizyme is the only known endogenous protein that binds to ornithine decarboxylase, inhibiting its activity and accelerating its degradation. Indeed, when tested in vitro, agmatine inhibited DNA synthesis and proliferation in some cell lines[10]. Moreover, Regunathan et al[11] reported that agmatine inhibited proliferation of human coronary artery vascular smooth muscle cells by stimulation of imidazoline rece-ptors. Satriano et al[12] claimed that agmatine dramatically decreased the ratio of DNA synthesis on mouse kidney proximal tubule cells by attenuation of the cellular polyamine level. In 2003, Gardini et al[13] found that agmatine inhibited the proliferation of rat hepatoma cells. These results indicate that agmatine might be an endogenous anti-proliferation factor, and whether the pharmacological effect of exogenous agmatine on cells in vivo is the same as in vitro is an interesting question.
In the present study, we investigated the inhibitory effects of agmatine on several classical tumor cells in vivo and in vitro and explored its possible mechanisms in vitro.
Materials and methods
Reagents and drugs Agmatine sulfate was obtained from the Beijing Institute of Pharmacology and Toxicology; cyclophosphamide was manufactured by Hengrui Pharmaceutical Co (Lianyungang, Jiangsu, China); spermine, spermi-dine, putrescine, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), and sodium dodecylsulfonate (SDS) were obtained from Sigma Chemical (St Louis, MO, USA); Roosevelt Park Memorial Institute medium (RPMI-1640) was purchased from Gibco (Carlsbad, CA, USA); and [3H]thymidine was obtained from DuPont/NEN Company (Boston, MA, USA).
Animals Male Kunming, Balb/c, and C57 mice [20±2 g, Grade II, Certificate No SCXK (Jun) 2002-001, Experimental Animal Center of Academy of Military Medical Sciences] were used. After transplanted with tumor cells, animals were randomly distributed into different groups. The control group was administered with saline alone and the others were treated with different drugs. All drugs were dissolved in normal saline and freshly prepared on the experimental day. Both normal saline and agmatine were administered subcutaneously (sc) and cyclophosphamide was injected intraperitoneally (ip) in a volume of 10 mL/kg. All of the animals were housed and maintained in a temperature-controlled room (22 °C–24 °C) with free access to qualified food and water at all times.
Cell culture MCF-7 human breast cancer cells were maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS), 100 kU/L penicillin and 100 kU/L streptomycin. Cells were incubated at 37 °C in a humidified 5% CO2 atmosphere and subcultured every 3 d.
Evaluation of agmatine’s inhibitory effects on the growth of tumor cells in vivo S180 sarcoma and B16 melanoma cells were subcultured in the abdominal cavity of mice for 8 d. The resulting ascites were diluted with saline to form a suspension containing 2×1010 cells/L. Aliquots of cell suspensions (0.2 mL) were injected (sc) into the right armpit of the mice. From the 1st day after implantation, saline, agmatine (5–40 mg/kg, tid, sc), or cyclophosphamide (20 mg/kg, qd, ip) were administered for 10 d. At d 10, the animals were killed and the tumors were chipped from their armpits. The tumor weights (g) were measured and the mean tumor weight of every group was calculated. The anti-tumor activities of the drugs were determined by a comparison between the inhibitory ratios obtained from the treated groups and the control group. The inhibitory ratio of drugs was expressed as [(average tumor weights in saline group–average tumor weights in drug treated group)/(average tumor weights in saline group)×100].
Measurement of proliferation in vitro in [3H]thymidine incorporation assay Proliferation of the MCF cells was assessed by [3H]thymidine incorporation assay. Briefly, cells suspended in RPMI-1640 medium with 10% FBS were seeded into a 96-well cell culture plate (80 µL/well) at a density of 6000 cells/well. Then they were treated with saline (control), or different concentrations of agmatine (1, 10, 100, 200, 500, or 1000 µmol/L), respectively, at a volume of 20 µL. Drugs were added for a total period of 48 h and [3H]thymidine (3.7×104 Bq/well) was added at 36 h of incubation. The medium was removed and the cells were washed three times with phosphate-buffered saline and then twice with ice-cold 10% trichloroacetic acid. Fixed cells were then solubilized in 0.2 mol/L NaOH (100 µL/well) and sonicated for 15 min. After mixing with scintillant liquid (1 mL) for 24 h, an aliquot (90 µL/well) was used for scintillation counting. Then the radioactivity was determined with a Multi-purpose Scintillation Counter (Columbus Instruments, Columbus, OH, USA). The mean cpm value of every group was calculated. The anti-proliferation potency of the drugs was determined by a comparison between the inhibitory ratios obtained from the treated groups and the control. The inhibitory ratio of the drugs was expressed as [(average cpm value in control group–average cpm value in drug treated group)/(average cpm value in control group)×100%].
Measurement of proliferation in vitro Cell proliferation was also confirmed again by measuring with MTT assay based on the colorimetric measurement of formazan dye formed from MTT by mitochondrial dehydrogenases. Exponentially growing cells were plated at a seeding density of 7.5×104 cells/mL in 96-well plates (80 µL/well). Then they were treated with saline (control), or different concentrations of agmatine (100, 200, 500, or 1000 µmol/L), respectively, at a volume of 20 µL. After they were incubated with or without drugs for 44 h, 20 µL of MTT reagent (0.5 g/L) was added to each well. The plates were incubated at 37 °C for an another 4 h. At the end of the incubation, the formazan crystals formed by MTT metabolism were solubilized by the addition of 100 µL of 10% SDS to each well. After 16 h, the absorbance of the solubilized product was measured at 570 nm in a Micro-plate Reader (Molecular Devices Corporation, Sunnyvale, CA, USA). The anti-proliferation potency of the drug was determined by a comparison between the inhibitory ratios. The percentage of growth inhibition was calculated by comparison of the absorbance of the treated group versus the control [(average absorbance value in control group-average absorbance value in drug treated group)/(average absorbance value in control group)×100].
Lactate dehydrogenase release assay To assess whether the reduction of cell numbers was attributable to the cellular toxicity of polyamines or agmatine, we measured the release of lactate dehydrogenase (LDH) in cell medium after drug treatment. Cells were cultured at a seeding density of 7.5×104 cells/mL in 24-well plates (800 µL/well). They were treated with saline, a range of concentrations of agmatine, spermidine, or spermine, respectively, at a volume of 200 µL and incubated at 37 °C and 5% CO2 for 48 h. Then 0.8 mL of the supernatant of each well was used for analysis by an Automatic Biochemical Analyzer (Hitachi7020, Tokyo, Japan). The release of LDH from the treated cells was compared to the control.
Statistical analysis Data were expressed as mean±SD. SAS software (SAS Inc, Raleigh, NC) was used to conduct a one-way ANOVA. P<0.05 was considered statistically significant.
Results
Inhibitory effects of agmatine on the growth of S180 sarcoma tumor cell lines in Kunming and Balb/c mice In Kunming mice transplanted with S180 sarcoma tumor cell lines, the S180 cells grew well and the average tumor weight reached 1.6 g in the normal, saline-treated group. Meanwhile, cyclo-phosphamide, a clinically approved anticancer agent, exhibited significant anti-tumor activity. In the cyclophosphamide-treated group (20 mg/kg, qd, ip), the average tumor weight was only 0.7 g and the inhibitory ratio was 56.3%. Agmatine also exerted a remarkable inhibitory effect on tumor growth. In the agmatine-treated groups (5–40 mg/kg, tid, sc), the tumor weights were significantly reduced (n=27, P<0.05) in a dose-dependent manner. The inhibitory ratio of tumor growth reached 31.3% at a dose of 40 mg/kg (Table 1).

Full table
In Balb/c mice transplanted with S180 sarcoma tumor cell lines, we obtained similar results. The average tumor weight was 1.0 g in the normal, saline-treated group. Cyclophosphamide (20 mg/kg, qd, ip) inhibited tumor growth significantly, and the inhibitory ratio reached 50.0%. Agmatine also inhibited the growth of tumors in a dose-dependent manner (n=10, P<0.05). The inhibitory ratio of agmatine on tumor growth was 50.0% at a dose of 40 mg/kg (Table 2).
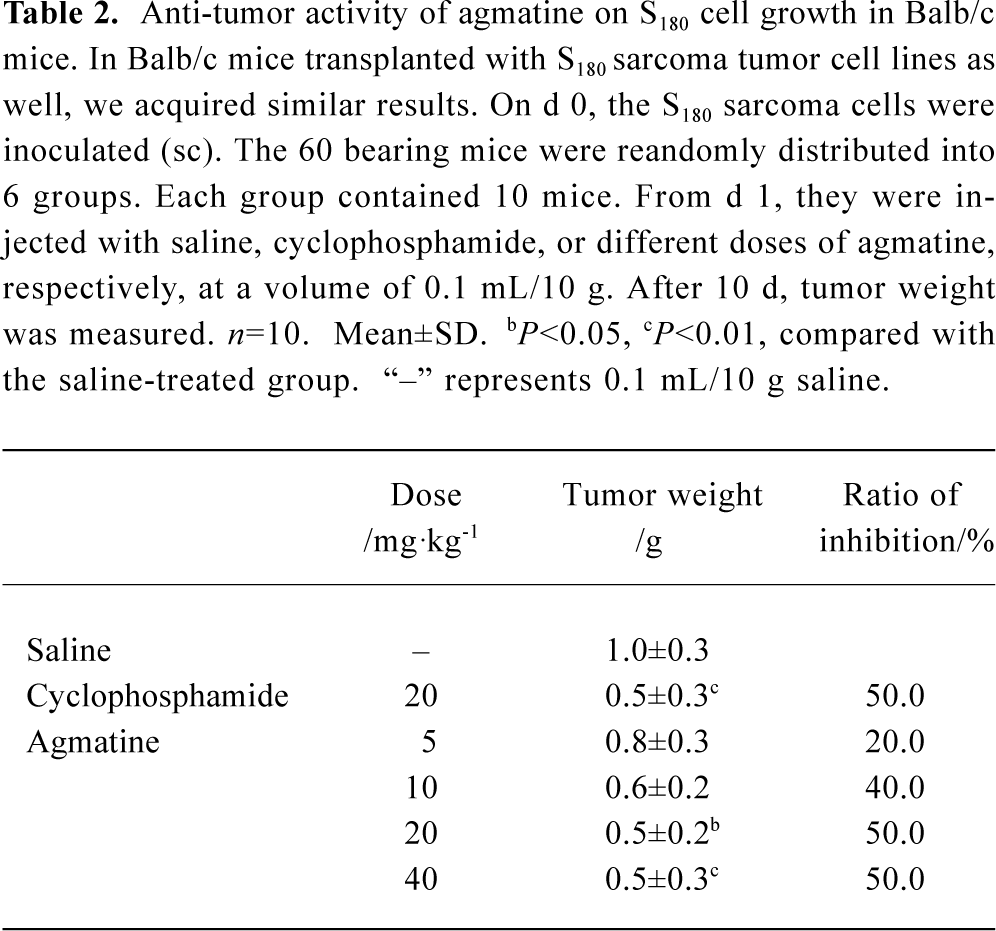
Full table
Inhibitory effects of agmatine on the growth of B16 melanoma tumor cell lines in C57 mice In C57 mice transplanted with B16 melanoma tumor cells, the tumor weight was 1.8 g in the normal, saline-treated group. In the cyclophosphamide-treated group (20 mg/kg, qd, ip), the tumor weight decreased to 1.0 g and the inhibitory ratio reached 44.4%. Agmatine (2.5–20.0 mg/kg, tid, sc) significantly suppressed the growth of the tumor (n=10, P<0.05), but the effect did not exhibit an obvious dose-dependent relationship (Table 3).

Full table
Inhibitory effect of agmatine on the proliferation of MCF cells in vitro In the [3H]thymidine incorporation assay, the MCF cells grew well and the average cpm value was 3143.8 in the normal, saline-treated group after a 48-h incubation. Agmatine showed anti-proliferation activity compared with the saline-treated group in a concentration-dependent manner. The cpm value was significantly reduced (n=8, P<0.05) after pretreatment with agmatine (1–1000 µmol/L). The inhibitory ratio of cell proliferation was 50.3% at a concentration of 1000 µmol/L (Table 4).

Full table
This effect of agmatine on cellular proliferation was further proved with the MTT assay. The MCF cells grew well and the absorbance value was 0.99 in the normal, saline-treated group after a 48-h incubation. Agmatine showed anti-proliferation activity compared with the saline-treated group in a concentration-dependent manner. The absorbance value was significantly reduced (n=8, P<0.05) after the cells were administered with agmatine (100–1000 µmol/L). The inhibitory ratio of cell growth was 23.8% at a concentration of 1000 µmol/L (Table 5).

Full table
We then investigated the time-dependent effect of agmatine on MCF cell proliferation. MCF cells were cultured for different lengths of time in the presence of 1 mmol/L agmatine, and cell viability was evaluated by a [3H]thymidine incorporation assay. Over 48 h, the inhibitory potency of agmatine strengthened gradually with prolonged time (n=8, P<0.01). The inhibitory ratio was 10%, 17%, 38%, and 62% at 12 h, 24 h, 36 h and 48 h, respectively (Figure 1).
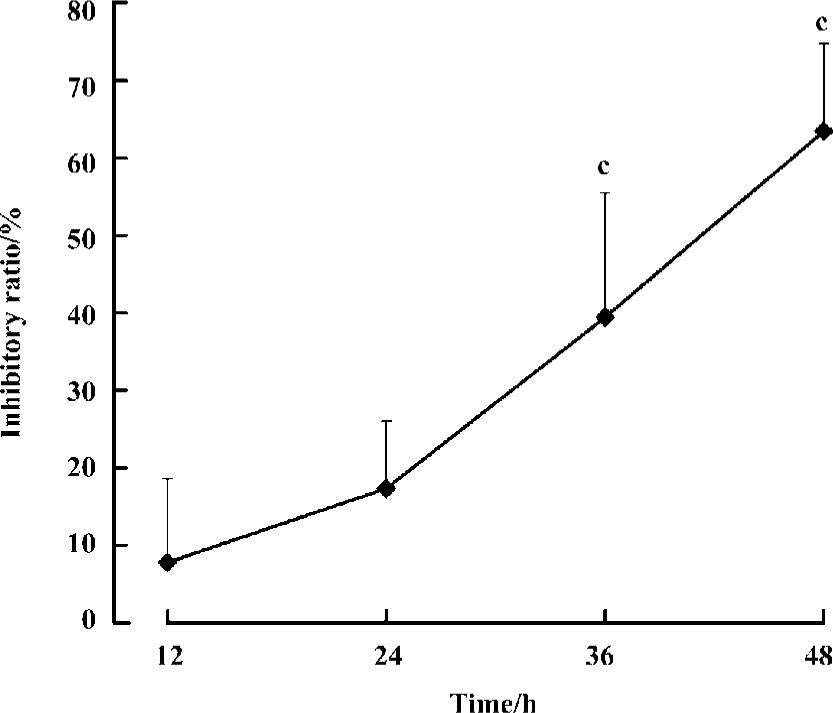
Effect of agmatine on LDH release in the medium To assess whether the reduction of cell numbers was attributable to toxicity of polyamines or agmatine, we measured the release of LDH in cell medium after drug treatment. Administration on MCF cells for 48 h, spermine (20 µmol/L) or spermidine (20 µmol/L) significantly increased the activity of LDH in the medium, but agmatine (1–1000 µmol/L) did not. At a concentration of 1000 µmol/L, agmatine decreased the LDH activity significantly (n=8, P<0.05; Table 6).

Full table
Putrescine reverses agmatine’s inhibitory effect on MCF cell proliferation As polyamines are essential growth factors, their dramatic intracellular decrease may be the main mechanism of the anti-proliferation action of agmatine. To check whether this is the mechanism involved, MCF cells were treated simultaneously with agmatine (1 mmol/L) and putrescine (12.5–100.0 µmol/L). Putrescine counteracted the inhibitory effect of agmatine on MCF cell proliferation in a concentration-dependent manner (n=8, P<0.05; Table 7).

Full table
Discussion
The present study demonstrated the inhibitory properties of agmatine toward S180 sarcoma and B16 melanoma cells in vivo. We found that, at doses of 5–40 mg/kg, agmatine suppressed S180 and B16 cell growth in three kinds of mice in vivo. The highest inhibitory ratio was more than 50.0%. We applied for a Chinese patent with these results in 2002 (02125495.8). Although Gardini et al reported that agmatine inhibited the proliferation of rat hepatoma cells in vitro by modulation of polyamine metabolism in 2003[13], they did not report anything related to the inhibitory effects of agmatine on the growth of transplanted tumors in vivo; in addition, the paper by Gardini et al[13] was published much later than when we applied for the Chinese patent. It is reasonable to state, therefore, that our current results demonstrate for the first time that agmatine has an inhibitory effect on S180 and B16 cell lines in vivo.
Polyamines play an essential role in proliferation, differentiation, and neoplastic transformation in mammalian cells[1]. Indeed, cellular polyamine levels are higher in tumor cell lines. Conversely, the depletion of polyamines results in growth arrest of neoplastic cells in vitro. The polyamine-biosynthetic pathway is an inviting target for the development of agents inhibiting carcinogenesis and tumor growth. The present therapeutic agents acting on this pathway are α-difluoromethylornithine (DFMO) and polyamine analogs[2]. They influence both polyamine synthesis and degradation and are now being used in clinical trials.
Agmatine is an analog of polyamines and can modulate the cellular concentration of polyamines[14]. As an intermediate of putrescine, agmatine may be a factor for increasing the cellular concentration of polyamines. Although tumor cells and tissues have been reported to have increased polyamines levels compared with normal cells, this increase is often in the range of 2- to 3-fold. When putrescine levels are approximately 10-fold higher than those present in cancer cells, the cells undergo apoptosis[15]. It has also been reported that overloaded polyamines have toxic effects on some normal cells. The toxicity of polyamines was studied in a well-characterized neuronal system of cerebellar granule cells in vitro. Twenty-four-hour exposure to spermine (1–500 µmol/L) resulted in a concentration-dependent death of granule cells, with the half of lethal dose (LD50) being reached at a concentration below 50 µmol/L. Putrescine was moderately toxic, with the LD50 at a concentration of only 500 µmol/L. The LD50 of spermidine was tested between concentrations of 50 and 100 µmol/L and its toxicity has been evaluated to be approximately 50% of that of spermine[16]. This was consistent with our results (data not shown). In contrast, agmatine has been postulated to decrease the cellular level of polyamines by inducing antizyme, competing with putrescine on transporter and other mechanisms.
So, in theory, agmatine might have double-edged effects on cell growth. But, to date, there has been no report that agmatine can enhance the proliferation of cells. Conversely, there is much evidence that agmatine can suppress cell proliferation, including different cells and malarial para-sites[17,18]. Consistent with these studies, the present study proves agmatine has a significant inhibitory effect on cell proliferation in several classical solid tumors in a transplanted model in vivo and in an MCF model in vitro. Putrescine prevented the effect of agmatine on [3H]thymidine incorporation in MCF cells. This effect of agmatine is similar to that of DFMO, which can block the synthesis of polyamines significantly. These results suggest that the effect of agmatine might be related to its influence on the synthesis of polyamine.
In addition, polyamines can interact with DNA direct-ly[19], so, in our experiments, the decreased [3H]thymidine incorporation may be caused by the inhibitory synthesis of DNA. As putrescine plays a partial role in energy supply[20], the results of the MTT assay indicate that agmatine might inhibit MCF cell proliferation by influence on energy metabolism. In addition, the effect on LDH activity in the medium showed that agmatine (1–1000 µmol/L) exhibited no cellular toxicity, whereas spermidine (20 µmol/L) and spermine (20 µmol/L) did. To sum up, these results partly demonstrate that agmatine does not increase the level of cellular polyamines and supports the conclusion that the anti-proliferation effect of agmatine is a result of polyamine limitation.
Currently, we know that agmatine’s toxicity is low and its effects on tumor cells are not similar to those of classical chemotherapeutical drugs. Regarding its low toxicity, its enhancement of opioid analgesia, and its antidepressant effect[21,22], we hope that agmatine could efficiently improve life quality of cancer patients. There are still many issues to be explored, and further experiments should be carried out to confirm our results.
In conclusion, agmatine has significant inhibitory effects on transplanted tumor growth in vivo and proliferation of tumor cells in vitro. The possible mechanisms might be related to inducing decrease of intracellular polyamine contents.
References
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem 1984;53:749-90.
- Seiler N. Pharmacological properties of the natural polyamines and their depletion by biosynthesis inhibitors as a therapeutic approach. Prog Drug Res 1991;37:107-59.
- Raasch W, Regunathan S, Li G, Reis DJ. Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci 1995;56:2310-30.
- Gabrielson EW, Pegg AE, Casero RA Jr. The induction of spermidine/spermine N1-acetyl transferase (SSAT) is a common event in the response of human primary non-small cell lung carcinomas to exposure to the new anti-proliferation polyamine analogue N1, N11-bis (ethyl) norspermine. Clin Cancer Res 1999;5:1638-41.
- Satriano J, Kelly CJ, Blantz RC. An emerging role for agmatine. Kidney Int 1999;56:1252-3.
- Vargiu C, Cabella C, Belliardo S, Cravanzola C, Grillo MA, Colombatto S. Agmatine modulates polyamine content in hepatocytes by inducing spermidine/spermine acetyltransferase. Eur J Biochem 1999;259:933-8.
- Cabella C, Gardini G, Corpillo D, Testore G, Bedino S, Solinas SP, et al. Transport and metabolism of agmatine in rat hepatocyte cultures. Eur J Biochem 2001;268:940-47.
- Satriano J, Isome M, Casero RA Jr, Thomson SC, Blantz RC. Polyamine transport system mediates agmatine transport in mammalian cells. Am J Physiol Cell Physiol 2001;281:C329-34.
- Gardini G, Cabella C, Cravanzola C, Vargiu C, Belliardo S, Testore G, et al. Agmatine induces apoptosis in rat hepatocyte cultures. J Hepatol 2001;35:482-9.
- Li G, Regunathan S, Barrow CJ, Eshragi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine displacing substance in the brain. Science 1994;263:966-9.
- Regunathan S, Reis DJ. Stimulation of imidazoline receptors inhibits proliferation of human coronary artery vascular smooth muscle cells. Hypertension 1997;30:295-300.
- Satriano J, Matsufuji S, Murakami Y, Lortie MJ, Schwartz D, Kelly CJ, . Agmatine suppresses proliferation by frameshift induction of antizyme and attenuation of cellular polyamine levels. J Biol Chem 1998; 273: 15 313–5.
- Gardini G, Cravanzola C, Autelli R, Testore G, Cesa R, Morando L, et al. Agmatine inhibits the proliferation of rat hepatoma cells by modulation of polyamine metabolism. J Hepatol 2003;39:793-9.
- Ishizuka S, Cunard R, Poucell-Hatton S, Wead L, Lortie M, Thomson SC, et al. Agmatine inhibits cell proliferation and improves renal function in anti-Thy-1 glomerulonephritis. J Am Soc Nephrol 2000;11:2256-64.
- Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanism and therapeutic applications. Cell Mol Life Sci 2001;58:244-58.
- Sparapani M, Dall’Olio R, Gandolfi O, Ciani E, Contestabile A. Neurotoxicity of polyamines and pharmacological neuroprotec-tion in cultures of rat cerebellar granule cells. Exp Neurol 1997;148:157-66.
- Su RB, Wei XL, Liu Y, Li J. Antimalarial effect of agmatine on plasmodium berghei K173 strain. Acta Pharmacol Sin 2003;24:918-22.
- Babal P, Ruchko M, Campbell CC, Gilmour SP, Mitchell JL, Olson JW, et al. Regulation of ornithine decarboxylase activity and polyamine transport by agmatine in rat pulmonary artery endothelial cells. J Pharmacol Exp Ther 2001;296:372-7.
- D’Agostino L, Di Luccia A. Polyamines interact with DNA as molecular aggregates. Eur J Biochem 2002;269:4317-25.
- Rustenbeck I, Eggers G, Reiter H, Munster W, Lenzen S. Polyamine modulation of mitochondrial calcium transport. Biochem Pharmacol 1998;56:977-85.
- Su RB, Li J, Qin BY. A biphasic opioid function modulator: agmatine. Acta Pharmacol Sin 2003;24:631-6.
- Li YF, Gong ZH, Cao JB, Wang HL, Luo ZP, Li J. Antidepressant-like effect of agmatine and its possible mechanism. Eur J Pharmacol 2003;469:81-8.
