Transfection of promyelocytic leukemia in retrovirus vector inhibits growth of human bladder cancer cells1
Introduction
Promyelocytic leukemia (PML), which encodes a growth transformation suppressor[1] and pro-apoptosis factor, mediating cell death by apoptosis[2], is disrupted by 15:17 chromosomal translocation in acute promyelocytic leukemia. The physical function of the PML gene was complicated and the PML gene has an altered expression during human onco-genesis. Its expression was reduced dramatically when cancer turned invasive[3]. Cells deficient in PML are resistant to apoptosis by multiple apoptotic stimuli, suggesting that PML is a pro-apoptosis factor[2]. Overexpression of PML protein could inhibit various human tumor growth in vitro and in vivo, such as in Hela cell[4], prostate cancer cells (PC-3,DU145, LNCaP)[5], breast cancer[6], and bladder cancer cells (5637; UM-UC-2 unpublished data)[7]. This evidence suggests that PML could be a candidate gene for human tumor gene therapy.
In this study, we further detected whether the PML as a growth suppressor could be a potential candidate gene for bladder cancer gene therapy. We constructed a recombinant retrovirus carrying the PML gene, which was controlled by long terminal repeat (LTR) promoter, and we want to study whether recombinant PML retrovirus has a high level PML protein expression in bladder cancer UM-UC-2 cells and whether overexpression of PML could inhibit bladder cancer cell growth.
Materials and methods
Cell culture and reagents UM-UC-2 cells[8] were cultured in Dulbecco’s modified Eagle’s medium (DMEM) at 37 °C supplemented with 10% fetal calf serum (FCS). PA317 packaging cells and NIH3T3 cell were maintained in RPMI- 1640 medium supplemented with 10% fetal bovine serum (FBS). Each medium was supplemented with penicillin (50 kU/L) and streptomycin (50 kU/L). The GST-PML antibody and PGS5-PML vector were obtained from Dr K S CHANG (MD Anderson Cancer Center, The University of Texas, USA). The PLXSN vector was purchased from Clontech Company.
Construction and amplification of pLPMLSN The PML retroviral vector pLPMLSN was constructed by inserting the full-length PML cDNA[1] into the EcoR I and BamH I restriction site of the pLXSN retroviral vector. The reconstructed plasmid DNA used for transfection was purified by the plasmid purification kit (Huashun Inc, Shanghai, China) according to the manufacturers’ instruction. DNA concentration was determined by UV spectrophotometer[4].
Generation of viral particles and infection to target cells pLXSN and pLPMLSN were transfected into the PA317 packaging cell lines by calcium phosphate coprecipitation[9]. PA317 packaging cells were split and grew in the selective medium with 800 mg/L of G418. After 2 weeks, G418 resistant colonies were picked and expanded in medium with 200 mg/L G418 and the supernatant viral particles were collected. The titer of viral stocks was calculated by infection of mouse fibroblast NIH 3T3 cell[11]. The titer of pPMLSN and pLXSN retrovirus was 1.3×109 CFU/L and 2.1×109 CFU/L, respec-tively.
The UM-UC-2 cells, as target cells, were infected with recombinant PML retrovirus and the control virus respectively by the supernatant gene transfer technique, as previously described[10]. In brief, UM-UC-2 cells in exponential growth were plated in 60-mm culture dishes 1 day before recombinant virus infection. Each culture dish was then fed with 10 mL of recombinant viral supernatant containing polybrene at a final concentration of 8 mg/L. The UM-UC-2 cells were then infected with viral particles for 48 h. The viral supernatant was prepared by incubating fresh medium for 1 day with the growth-accelerated viral producer and then used directly for further experiments.
Identification of PML cDNA in transfected cells Genomic DNA was prepared from UM-UC-2, UM-UC-2/pPMLSN, UM-UC-2/pLXSN cells and used for polymerase chain reaction (PCR) with primers specific for PML (forward 5'-CTT GAA CCT CCT CGT TCG ACC-3' reverse 5'-GTA CAA CAG GTA GCG GAT CCC-3'). The forward primer was specifically designed for pLXSN vector, and the reverse primer was for PML cDNA. The reaction mixture for PCR amplification was subjected to 30 cycles of denaturation (95 ºC, 60 s), annealing (56 ºC, 60 s), and extension (72 ºC, 60 s). The amplification products were identified by 2% agarose-gel electrophoresis.
Identification of PML protein expression in transfected cells by immunofluorescence staining and Western blot Western blot and immunofluorescence staining of bladder cancer UM-UC-2 cells infected with recombinant PML retrovirus and control virus were carried out as described in our previous report[5]. Briefly, UM-UC-2 cells were seeded on coverslides for at least 4 h, and then recombinant PML retrovirus was added and incubated at 37 ºC for 24 h. Cells were washed in phosphate-buffered saline (PBS) twice and fixed in 4% paraformaldehyde for 20 min and 0.1% Triton X-100 for 10 min, followed with three washes in PBS containing 0.1% BSA. Cells were then incubated in the washing buffer for 20 min at room temperature. Immunofluorescence staining was performed using the affinity-purified antipeptide polyclonal antibody GST-PML at dilution of 1:2000 for 1 h at 37 ºC, and then goat anti-rabbit IgG-fluorescein isothiocya-nate secondary antibody (Sabc Inc, Beijing, China) was added for another 1 h. Confocal microscopy analysis was performed by using a Zeiss (New York, NY, USA) laser scanning confocal microscope at 494-nm stimulated wavelength.
Western blot of PML protein was performed using RIPA lysis buffer and 8% SDS-PAGE gel electrophoresis. Proteins were transferred to NC filter (Bio-Rad) and blocked with 5% non-fat milk for 1.5 h. The filters were then incubated for 2 h with a dilution of 1:2000 GST-PML antibody and then for 1 h with the secondary antibody at dilution of 1:500. Immuno-detection was performed using the ECL Western blot detection system.
Cell viability UM-UC-2 Cell viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assay. In brief, we selected 1 d, 2 d, 3 d, and 4 d as different times for observation points. Bladder cancer cells (1×104) were plated in 96-well tissue culture plates in DMEM containing 10% FBS in a final volume of 0.2 mL. When the cells reached 50% confluence, they were treated with recombinant PML retrovirus and control virus. After 24 h of culture, cell proliferation was assessed by directly adding 50 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide 7 dye (0.005 mg/L) to the medium for another 4 h, and then bladder cancer cells were solubilized in Me2SO (150 µL/well) on a shaker at room temperature for 10 min before reading the absorbance at 570 nm using a Biorad Technologies Microplate Reader.
Statistical analysis The experimental results shown were repeated three times, unless otherwise indicated. Results are expressed as mean±SD. Statistical analysis was carried out using Student’s t-test and one-way ANOVA. Significance was set at P<0.05. Statistical analyses were performed using SPSS10.0 (SPSS Inc, Chicago, IL, USA).
Results
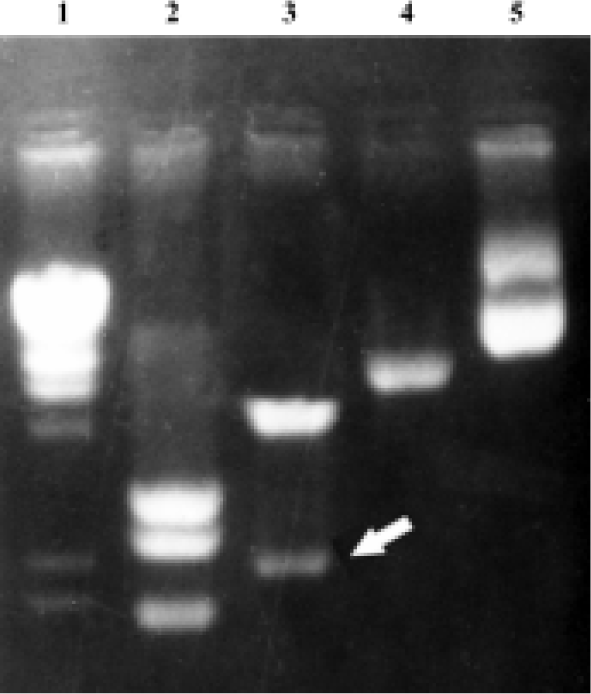
Identification of the recombinant retrovirus vector pLPMLSN The full-length PML cDNA was excised by EcoR I and BgL II from the plasmid pSG5PML[1], and then ligated into the EcoR I and BamH I digested restriction site, for BgL II and BamH I are isocaudamers, which have the same cohesive terminus. The constructed plasmid was digested with Kpn I, and three fragments of 2.0 kb, 2.9 kb, and 3.1 kb are acquired on agarose-gel electrophoresis. Two 2.3 kb and 5.7 kb fragments were obtained by EcoR I and Hind III (Figure 1). It suggests that PML cDNA had been inserted into retrovirus vector pLXSN successfully.
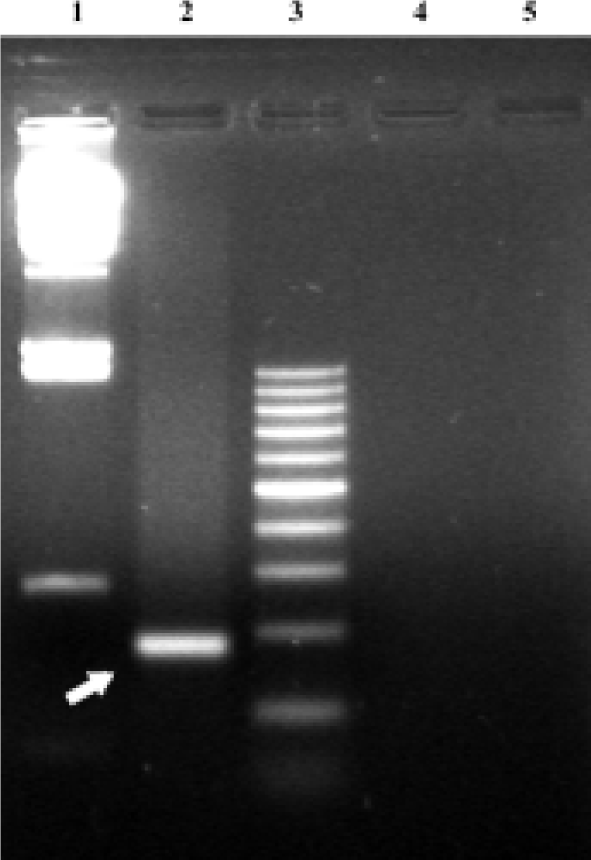
Identification of PML cDNA in transfected cells PCR was performed using total DNA samples prepared from UM-UC-2 cell culture after the recombinant retrovirus particles were transducted into the cultured cells. The PCR products of 304 bp fragments for PML were amplified from UM-UC-2/pLPMLSN cells but not from control cells infected with UM-UC-2/pLXSN and UM-UC-2 parental cells (Figure 2).
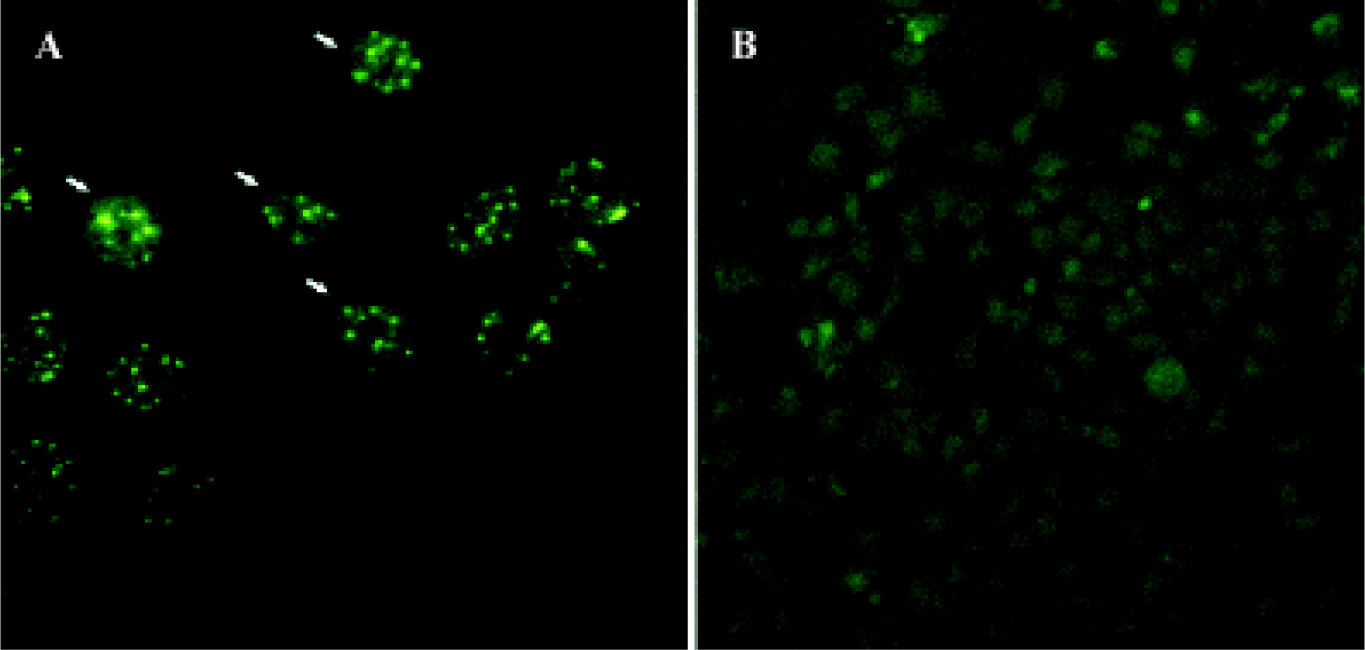
Determination of PML expression after recombinant PML retrovirus infection by immunofluorescence Immunofluorescence staining was performed to confirm whether the reconstructed retrovirus could infect and express PML protein in bladder cancer cells UM-UC-2. As shown in Figure 3, both the number and intensity of PML specific nuclear speckle significantly increased in UM-UC-2/pPMLSN cells, while in parental cells only a little poor and nonspecific signal was detected.
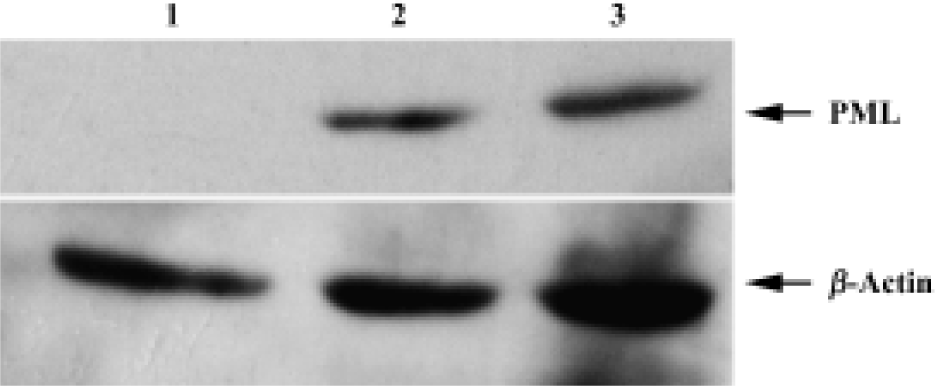
Demonstration of PML expression after recombinant PML retrovirus infection by Western blot Western blot analysis showed that the bladder cancer UM-UC-2 cells infected with recombinant PML retrovirus expressed a high level of 90 kDa PML protein. The protein was undetectable in the parental bladder cancer UM-UC-2 cells by Western blot (Figure 4).
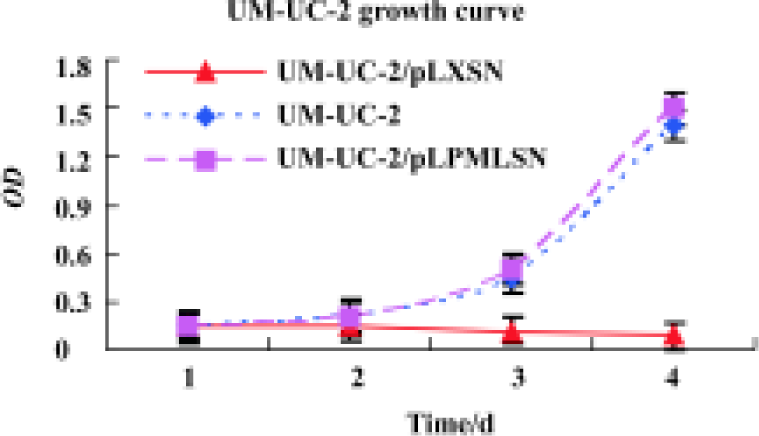
Effect of PML overexpression on cell growth We had previously shown that PML could repress the growth of prostate cancer LNcap, DU145, and PC-3 cells[5]. Now, to further test the effect of increased expression of PML on bladder cancer UM-UC-2 cell growth, MTT assay was used. As shown in Figure 5, UM-UC-2/pLPMLSN grew significantly more slowly than the parental UM-UC-2 and control UM-UC-2/pLXSN cells (P<0.05).
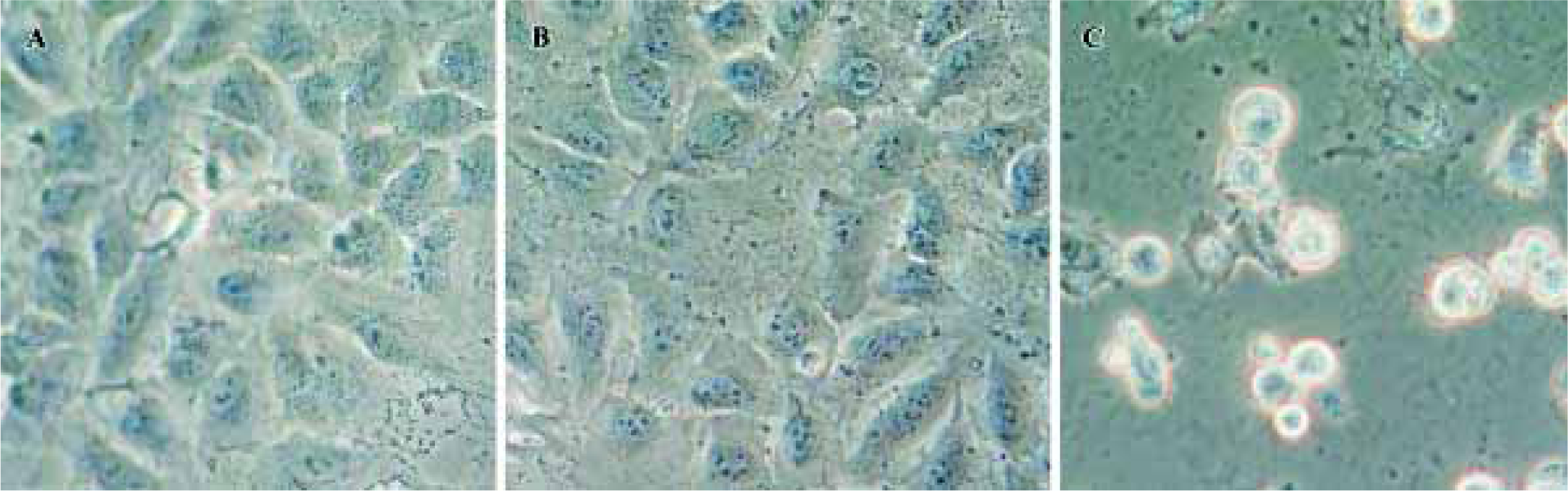
Morphological changes were also observed under phase contrast and light microscopes. As shown in Figure 6A and 6B, UM-UC-2 and UM-UC-2/pLXSN control cells showed normal morphology. In contrast, UM-UC-2/pLPMLSN cells were shrunk, lost cell-to-cell contact, and in part detached from the plate (Figure 6C). These characteristics suggested apoptotic morphology feature.
Discussion
In recent years, studies have been provided that activated oncogene or loss of tumor suppressor leads to the development of human cancer. Based on this concept, new effective therapeutic regimens have been developed as alternatives to conventional cancer therapy. One field that most scientists focused on is gene therapy, which is to suppress the overexpressed oncogene or rebuild the function of tumor suppressor[12]. To deliver a gene into tumor cells, two major vector systems, such as viral system and non-viral system, have been thrown into research studies and clinical trials. The latter has relative low transduction efficacy and required some special equipment, therefore was not widely used. Compared to the adenovirus system, the retrovirus systems have some advantages in tumor gene therapy because of the following reasons. First, infection by retrovirus depends on host cell division. Most tumor cells are active in cell mitosis, which made wide range infection to carrier cells[13]. Second, retrovirus can integrate into host genome, which can stably and continuously express targeting protein as host cell growth[13]. Third, a recent study demonstrated that the entrance of adenovirus to the host cell must be mediated by the specific receptor. For example coxsackie and adenovirus receptor (CAR), whose protein expression level is reduced and even lost when breast cancer[14], urothelial cancer[15,16], head, and neck cancers[17] are in high-grade or become invasive as adenovirus mediated gene therapy is not adapt to high-grade, late-stage invasive tumor[14–17]. While the retrovirus does not have these limitations. This is one good reason why we constructed the recombinant PML retrovirus. Finally, when adenovirus infects the host cells for gene therapy, frequent administration of recombinant adenovirus were required to achieve therapeutic efficacy. Because of a strong immunological response, it could not operate repeatedly[5]. In contrast, the retrovirus did not have the adverse immunological response.
We constructed recombinant retrovirus vector carrying PML gene, which was involved in the 15:17 translocation in acute promyelocytic leukemia (APL), and is a growth and transformation suppressor and plays an essential role in multiple pathways of apoptosis[1,2]. PML is lost or reduced in solid tumor of several histological origins[4–7] and is found to be more frequently lost in late-stage or metastatic cancer[3]. Our previous study demonstrated that endogenous PML expression was related to the stage, grade, and invasiveness of bladder cancer. Stably overexpressed PML protein leads to decreased cell growth and tumorigenicity of bladder cancer in vivo[7] and prostate cells in vitro and in vivo[5]. Overexpression of PML induced G1 cell cycle arrest and subsequently triggered cell death by apoptosis in breast cancer cells[6].
In addition, PML is a much more stable protein with long half-life of about 6 h compared to other tumor suppressor genes such as p53 which has a half-life of 1.5 h. This suggest that long-time and high concentration of protein could accumulate in tumor cells and a better therapeutic effect could be obtained[5].
These results strongly indicate that PML has therapeutic potential in the gene therapy of human tumors.
In conclusion, we constructed recombinant retrovirus stable expressed PML. PCR confirmed that the recombinant retrovirus could infect bladder cancer cells. Laser scanning confocal microscope and Western blot showed high level PML protein could be expressed. Overexpression of PML could inhibit the growth of bladder cancer. It may be an attempt for human tumor gene therapy in future.
References
- Mu ZM, Chin KV, Liu JH, Lozano G, Chang KS. PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol Cell Biol 1994;14:6858-67.
- Wang ZG, Ruggero D, Ronchetti S, Zhong S, Gaboli M, Rivi R, et al. PML is essential for multiple apoptotic pathways. Nat Genet 1998;20:266-72.
- Koken MH, Linares-Cruz G, Quignon F, Viron A, Chelbi-Alix MK, Sobczak-Thepot J, et al. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene 1995;10:1315-24.
- Mu ZM, Le XF, Vallian S, Glassman AB, Chang KS. Table overexpression of PML alters regulation of cell cycle progression in HeLa cells. Carcinogenesis 1997;18:2063-9.
- He D, Mu ZM, Le X, Hsieh JT, Pong RC, Chung LW, et al. Adenovirus-mediated expression of PML suppresses growth and tumorigenicity of prostate cancer cells. Cancer Res 1997;57:1868-72.
- Le XF, Vallian S, Mu ZM, Hung MC, Chang KS. Recombinant PML adenovirus suppresses growth and tumorigenicity of human breast cancer cells by inducing G1 cell cycle arrest and apoptosis. Oncogene 1998;16:1839-49.
- He D, Nan X, Chang KS, Wang Y, Chung LW. Overexpression of the promyelocytic leukemia gene suppresses growth of human bladder cancer cells by inducing G1 cell cycle arrest and apoptosis. Chin Med J 2003;116:1394-8.
- Grossman HB, Wedemeyer G, Ren L. UM-UC-1 and UM-UC-2: characterization of two new human transitional cell carcinoma lines. J Urol 1984;132:834-7.
- Jseph S, Daivd WR. Laboratory manual. 3rd ed. Cold Spring, NY: Cold Spring Laboratory; 2001. p 1282–6.
- Miller AD, Miller DG, Garcia JV, Lynch CM. Use of retroviral vectors for gene transfer and expression. Methods Enzymol 1993;217:581-99.
- Bodine DM, McDonagh KT, Seidel NE, Nienhuis AW. Development of a high-titer retrovirus producer cell line and strategies for retrovirus-mediated gene transfer into rhesus monkey hematopoietic stem cells. Ann N Y Acad Sci 1990;612:415-26.
- Friedmann T. Progress toward human gene therapy. Science 1989;244:1275-81.
- Sokol DL, Gewirtz AM. Gene therapy: basic concepts and recent advances. Crit Rev Eukaryot Gene Expr 1996;6:29-57.
- Shayakhmetov DM, Li ZY, Ni S, Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res 2002;62:1063-8.
- Li Y, Pong RC, Bergelson JM, Hall MC, Sagalowsky AI, Tseng CP, et al. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res 1999;59:325-30.
- Rauen KA, Sudilovsky D, Le JL, Chew KL, Hann B, Weinberg V, et al. Expression of the Coxsackie adenovirus receptor in normal prostate and in primary and metastatic prostate carcinoma: potential relevance to gene therapy. Cancer Res 2002;62:3812-8.
- Kasono K, Blackwell JL, Douglas JT, Dmitriev I, Strong TV, Reynolds P, et al. Selective gene delivery to head and neck cancer cells via an integrin targeted adenoviral vector. Clin Cancer Res 1999;5:2571-9.
